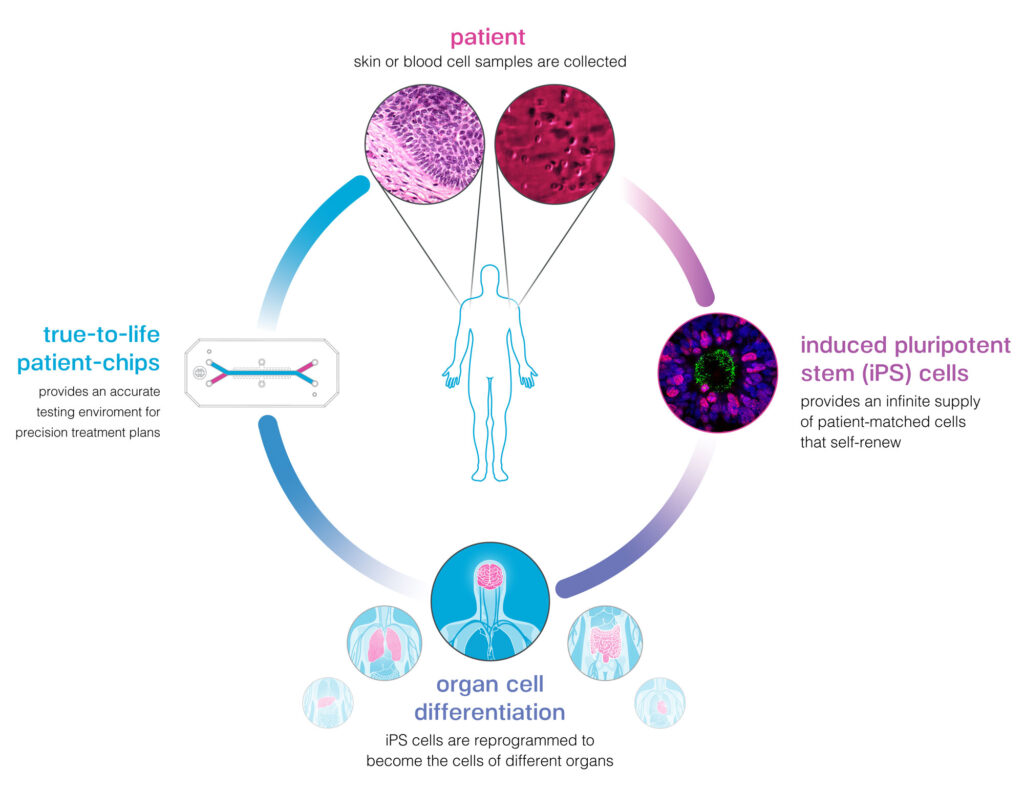
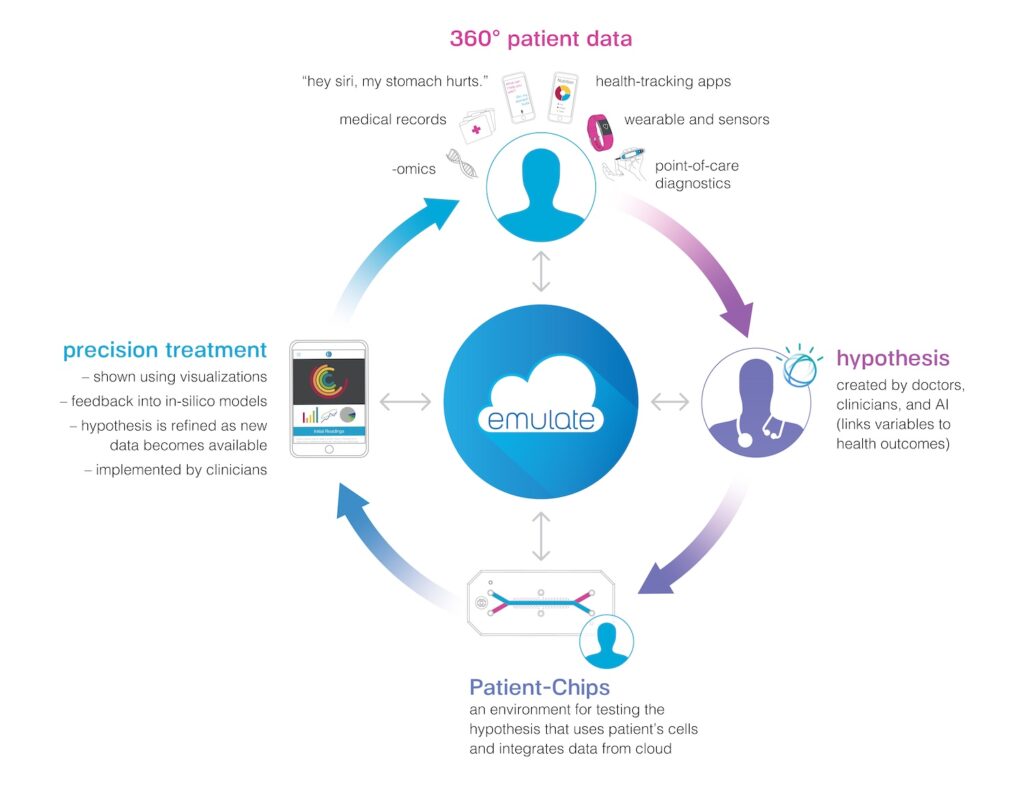
Our mission to improve human health is not limited to any race, gender, color, creed, or sexual orientation, and we cannot realize this mission without first acknowledging the inequities in our society and working towards racial equity and justice.
As a company, we believe the ongoing injustice of systemic racism and oppression in our society needs to be addressed. The deaths of George Floyd, Breonna Taylor, Tony McDade, Ahmaud Arbery, and Manuel Ellis—as well as the far too many unnamed Black men and women before them—are a tragedy and a result of centuries-old systemic oppression and racism that have hurt our nation and our Black communities.
As an organization, we stand with many others who honor the Black lives lost due to the devastating effects of racial inequality, racial injustice, and White supremacy. We are also united with common values as part of our company principles called “Being Human”—a set of guidelines created by our team to help us all work in harmony. At its core, “Being Human” is a shared belief that compassion, honesty, and empathy are essential in achieving healthy collaboration toward our mission. But we cannot fully realize this principle without acknowledging unequivocally that Black Lives Matter.
As an organization, we stand with many others who honor the Black lives lost due to the devastating effects of racial inequality, racial injustice, and White supremacy.
We realize that there is work to be done in order to address the rampant inequalities that permeate our society, and we are committing to doing our part.
This includes, but is not limited to:
From June 1, 2020 through December 31, 2020, we are pledging to match employee donations to the below local and national organizations:
We are working to create a more inclusive workplace so all Emulate employees feel safe, comfortable, and valued. This includes:
-
Ensuring our hiring and professional development policies to promote diversity and racial equity across our organization.
-
Implementing anti-racism education and trainings to our entire team with the help of outside diversity and inclusion professionals and anti-racism educators.
-
Ensuring there are anti-bias practices in our hiring process to proactively level the playing field for all candidates.
-
-
We are updating our time-off policies to support paid time-off for employees who choose to exercise their constitutional rights to protest or assemble for the sake of any social movement or gathering.
This is just the beginning. Our work will be ongoing as we continue to learn and grow as an organization, and we are committed to taking action to bring an end to injustice and inequality.