CEO, Jim Corbett, shares how new funding will enable Emulate to expand research and development efforts to new heights
In March 2020, I was fortunate to join Emulate, intrigued by one of those rare opportunities to bring truly game-changing technology to healthcare.
Five days later, the world shut down.
Every day since has been motivated by a vital mission to leverage human biology and technology to ignite a new era in human health, driven by a team of people with an unwavering commitment to bring a human-centric approach to biological innovation.
As we were coming to terms with our new normal, our team’s focus and action were nothing short of miraculous. Undaunted, and united under a single vision, the team persevered. We found new ways to move forward with a collective strength to overcome challenges. We embraced new processes to shatter unexpected roadblocks. And we responded to the needs of our customers to ensure their science could keep moving forward.
ONE Curious, Brave and Human Team
We grew our team, adding deep commercial and functional expertise to implement structure and processes in order to rapidly scale. The scientific team has grown to include researchers focused on our application roadmap areas such as immunology and microbiome. We expanded our leadership team with Donald Ingber, MD, PhD, a scientific founder of Emulate and Chairman of its Scientific Advisory Board – and arguably one of the great pioneers in the field of biologically inspired engineering – joining our Board of Directors. Also, Scott Kantor accepted the role of Chief Financial Officer and Larry Weiss came on as Chief Legal Officer.
Breakthrough Innovations that Challenge the Status Quo
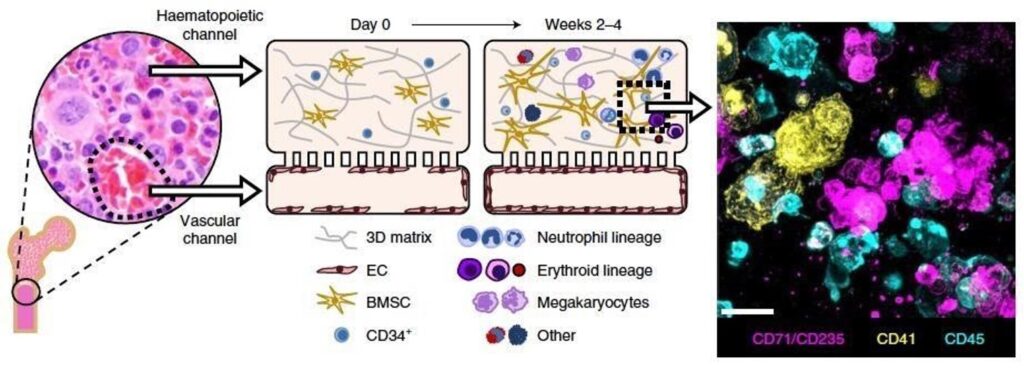
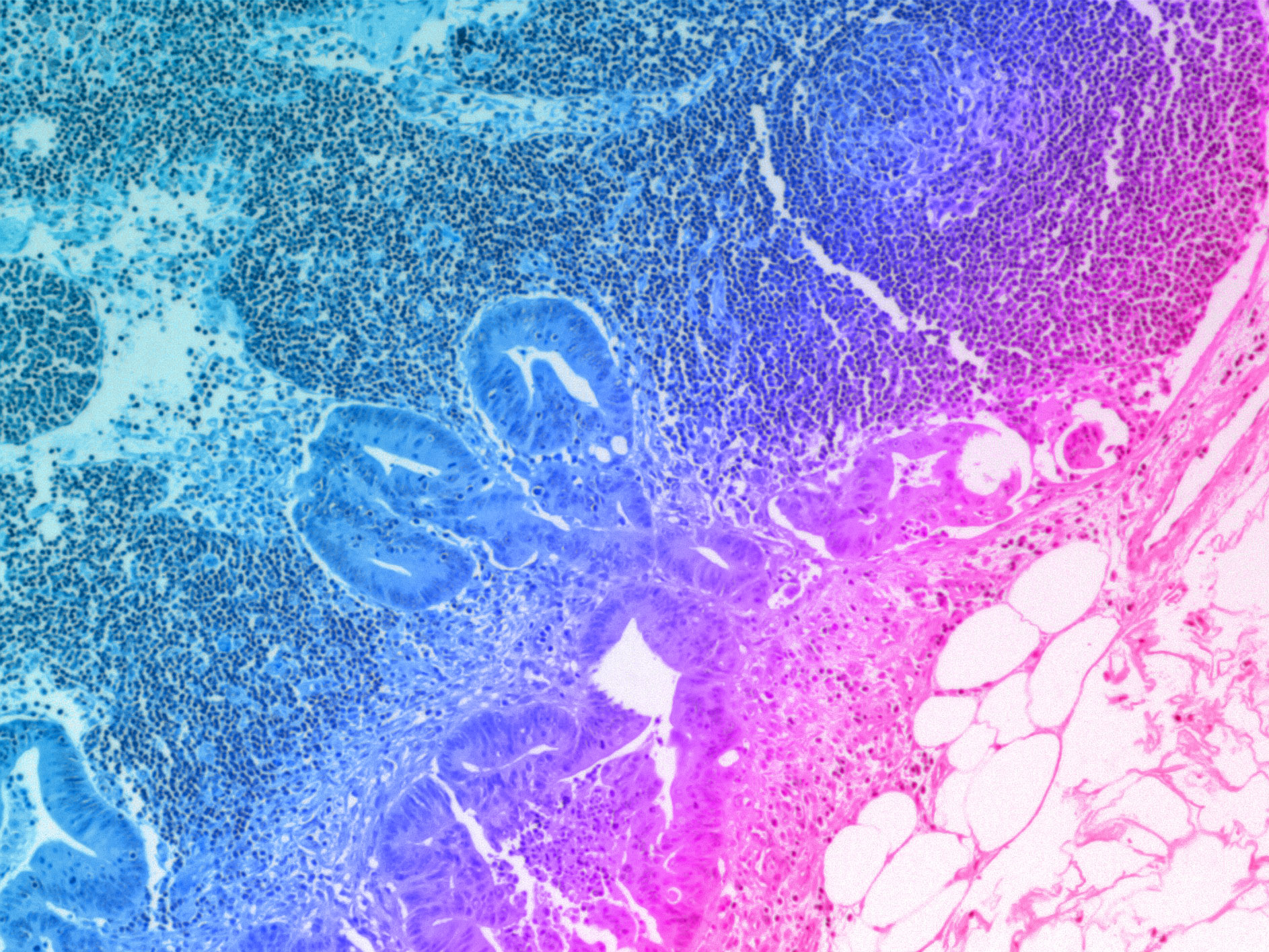
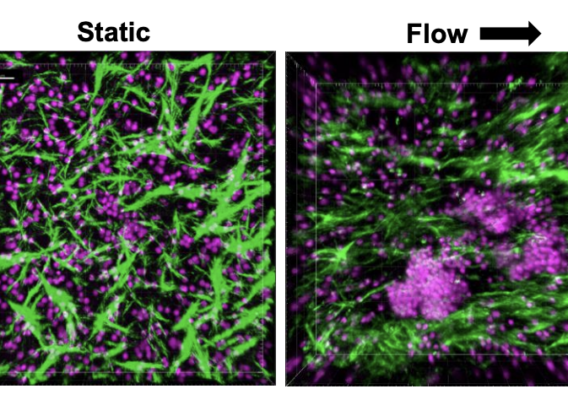
Over the past 18 months, we announced our roadmap for expanding Organ-Chip applications with a focus on developing targeted and advanced biologics. Additionally, we released a series of breakthrough applications and organ-models, such as the Brain-Chip and the Colon Intestine-Chip, deepening our portfolio of the human-relevant Organ-Chips that helped solidify our commitment to scientific excellence.
We have bolstered our leadership team, accelerated product development goals, and seen healthy growth in demand for our products. Several leading indicators validate our belief that organ-on-a-chip technology will dramatically transform the entire drug discovery and development pipeline and ultimately eliminate unnecessary animal testing.
Jim Corbett, Emulate CEO
Policy-Changing Influence and Advancements
Aside from our product and commercial developments, we have taken a stronger stance against animal testing, as there is scientific proof that it has significant limitations in effectiveness – particularly for today’s novel drugs and vaccines. In response to a New York Times article, we championed legislation reducing the use of primates for drug testing. More recently, the FDA Modernization Act and the Humane Research Act have been introduced to Congress to rally support for alternatives to animal testing, citing scientific inadequacies and ethical concerns associated with it. Our team also joined the North American 3Rs collaborative and believe that moving in the right direction means encouraging the use of more accurate and humane technologies. We truly believe that organ-chips will become an integral part of drug development and has the potential to ultimately eliminate animal testing.
Onward Movement
This level of achievement would be astonishing in normal times and no doubt helped propel our fundraising efforts. Today, we announced the close of an $82 million dollar round of financing, which will enable Emulate to expand research and development efforts to new heights. We will scale-up R&D activities to facilitate the creation of new human-relevant organ-on-a-chip models in immunology, neuroinflammation, tumor modeling, and more. To help meet growing global demand, we will extend operations in the Asia Pacific region with new distributor relationships.
I am excited to see what this team will accomplish as we set out into a changed world, continuing to scale and meet global demand as well as allow researchers to predict human response better than conventional cell culture and animal-based experimental testing. This biological revolution is just getting started. Let’s go!