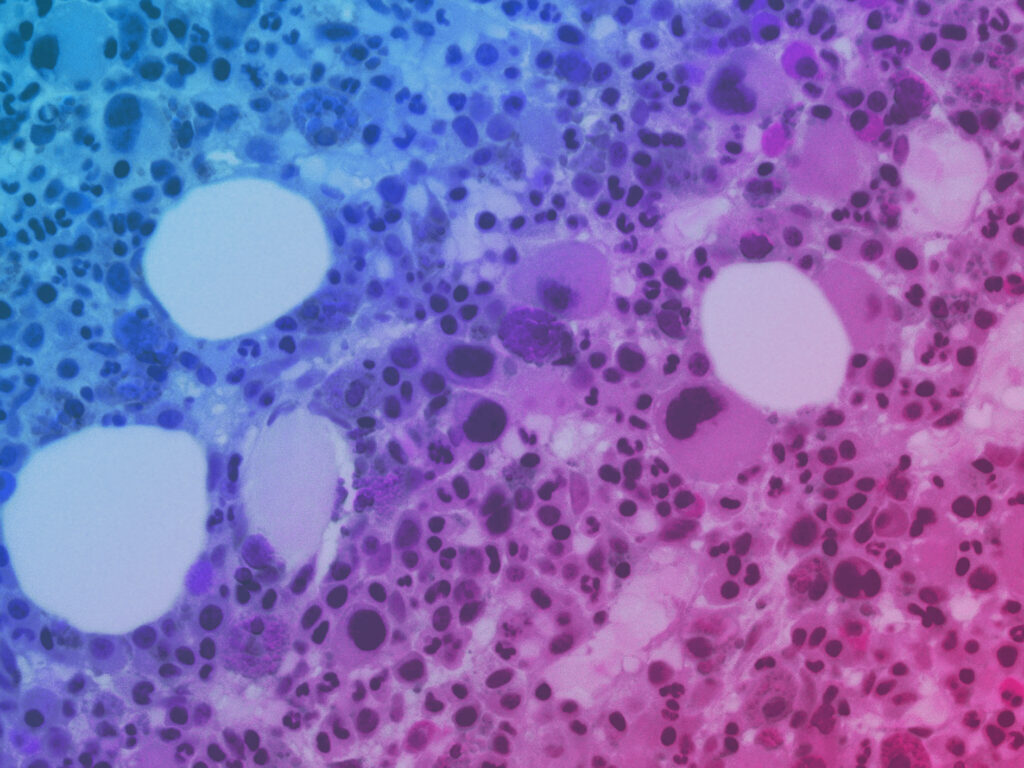
A summary of the paper published in Nature Biomedical Engineering: On-chip recapitulation of clinical bone marrow toxicities and patient-specific pathophysiology.
Human bone marrow is the body’s major site of hematopoiesis, the proliferation and differentiation of all blood cellular components from hematopoietic stem cells. These actively dividing and maturing cells are highly sensitive to toxic stimuli and, thus, bone marrow is one of the major targets of unwanted toxicity caused by drugs or therapeutic agents. Predicting drug-related hematotoxicity with clinically relevant pharmacokinetics would be extremely valuable for designing safety assessments and human trials.
Studying bone marrow tissue is particularly difficult due to its inaccessibility and the limited number of physiologically relevant in vitro models that can support growth and maintenance of bone marrow cultures. Current hematopoietic toxicity studies are performed using methylcellulose colony cultures in which cells are suspended and can be exposed to higher-than-usual concentrations of drugs for extended periods of time. To address the unmet need for a predictive model to study hematopoietic dysfunction and pathophysiology of bone marrow, the authors created a Bone Marrow-Chip (BM-Chip) to recapitulate relevant hematopoietic cell types in a three-dimensional (3D), mechanically active next-generation in vitro model. This model possesses relevant levels of complexity to closely mimic the in vivo environment to examine the effects of known myelotoxic stressors and to investigate a genetic condition resulting in bone marrow failure.
- Research Area: Drug development, Toxicity testing, Rare Disease
- Organisms: Human
- Sample Types: Human bone marrow-derived mesenchymal stromal cells, hematopoietic stem/progenitor (CD34+) cells, umbilical cord vascular endothelial cells
- Research Question: Can a Bone Marrow-Chip recapitulate the features of human hematopoiesis and dysfunction resulting from genetic mutations and exposure to drugs and radiation?
Experimental Overview
Characterizing the Bone Marrow-Chip
Researchers created the BM-Chip using human bone marrow-derived stromal cells (BMSC) and CD34+ cells co-cultured in a fibrin gel in the upper channel of the chip. The lower channel of the chip contained human umbilical vein endothelial cells (HUVEC) with unidirectional continuous flow of culture medium containing cytokines that support cell growth (stem cell factor, Flt3 ligand and thrombopoietin) as well as factors that encourage multilineage differentiation (granulocyte colony stimulating factor and erythropoietin). Compared to the same cells grown in static models in suspension and in 3D gel, the BM-Chip increased the numbers of proliferating myeloerythroid and neutrophil progenitors, demonstrating increased cell survival and robust hematopoiesis in vitro over prolonged culture time (28 days).
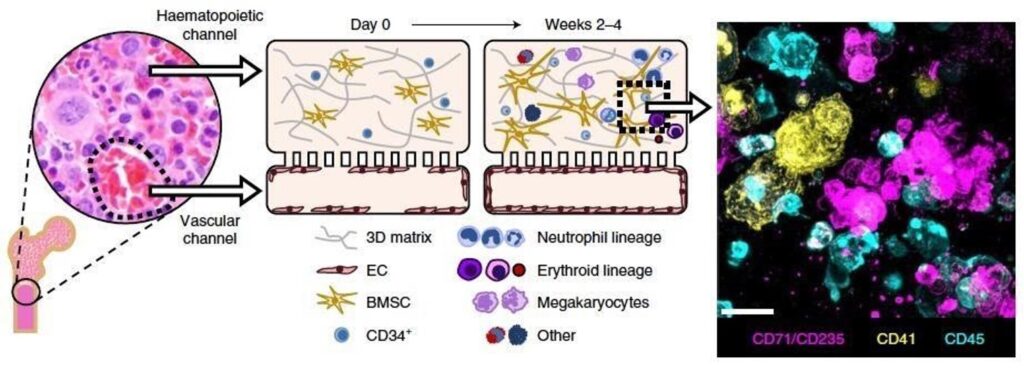
Schematic of bone and insert micrograph showing normal human bone marrow histology. Left middle: schematic of the cross-sectional view of the human BM-Chip on day 0 after seeding, showing singly dispersed CD34+ progenitors and BMSCs in a gel in the top channel and an incomplete vascular lining (seeded on either day 0 or day 8) in the bottom channel. Right middle: within 2 weeks of culture initiation, endothelial cells grow to cover all four sides of the lower channel and create a vascular lumen, while CD34+ cells undergo expansion and multilineage differentiation. Right: immunofluorescence image of a vertical cross-section through the gel in the upper channel of the BM-Chip taken at day 14 (magenta: erythroid lineage; yellow: megakaryocyte lineage; blue: neutrophil and other hematopoietic lineages).
Modeling Drug-Induced Hematotoxicity in the Bone Marrow-Chip
To determine if the BM-Chip could be used to model human bone marrow pathophysiology, the researchers exposed the chips to 5-fluorouracil (5-FU) at dosage-appropriate plasma concentrations to observe toxic effects. BM-Chips from six different CD34+ donors infused with 5-FU through the vascular (bottom) channel consistently showed hematotoxicity at clinically relevant concentrations, whereas suspension and static gel co-cultures did not.
In the next set of experiments, the researchers challenged the BM-Chip with AZD2811, a small-molecule inhibitor of Aurora B kinase that is critical for proper cell division and is currently in phase II clinical development as a cancer therapeutic drug. In earlier phases of clinical trials, AZD2811 has been shown to selectively target dividing neutrophil and erythroid cells with varying effects in a time and dose-dependent manner. The authors used the BM-Chip to explore these effects on both mature and progenitor cell types and provide insight into the drug’s mechanisms of action. 2- and 48-hour AZD2811 infusions resulted in dose-dependent toxicities of the erythroid lineage, with much greater effect following the 2-hour infusion regimen than the 48-hour regimen. In the neutrophil lineage, dose-dependent toxicity in the BM-Chip was greater with the 48-hour infusion. Importantly, both regimens revealed maturation-dependent sensitivity to the treatment via selective decrease in immature cells of both lineages compared to mature cells.
Suspension cultures of CD34+ cells failed to replicate the dose-dependent erythroid toxicity that was successfully modeled by the human BM-Chip, suggesting that it can more accurately predict hematological effects at clinically relevant drug exposures than static culture models.
Radiation and Bone Marrow Recovery
Building on the observation that immature, proliferating cell types were selectively affected by AZD2811 treatment, the authors tested maturation-dependent toxicity caused by ionizing radiation. Radiation treatments are widely used as a cancer therapy, and they commonly cause bone marrow depletion – a reduced production of blood cells from all lineages. The authors noted that the BM-Chips showed decreased numbers in all cell types studied that correlated with increasing doses of radiation. These experiments show that the BM-Chip is a useful model of human-specific radiosensitivity, inviting more detailed analyses of radiation-associated cell death.
The researchers went on to demonstrate that the BM-Chip can also be used as an in vitro model to examine bone marrow recovery and response to potential therapeutics using human-relevant pharmacokinetic profiles during preclinical drug evaluation, development, and regulatory assessment, which cannot be done using animal models.
Modeling Rare Conditions: Shwachman-Diamond Syndrome
Shwachman–Diamond syndrome (SDS) is a genetic condition that causes bone marrow depletion and failure, resulting in neutropenia. The extent of hematological dysfunction may involve other cell lineages as well, leading to myeloid malignancies in some SDS patients. Understanding SDS has been limited by a lack of animal models that faithfully recapitulate the condition. In this study, the authors cultured CD34+ cells from two patients with SDS alongside normal BMSCs and endothelial cells to understand the clinical manifestations of the syndrome using this disease model.
The researchers noted that the cells in the SDS BM-Chips exhibited defective hematopoiesis, with fewer numbers of neutrophils and erythroid cells, as well as impaired maturation of neutrophils. The blunted maturation of neutrophils, denoted by their pattern of CD16/CD13 expression, matched retrospective patterns found in bone marrow aspirates of other SDS patients, suggesting that the BM-Chip can replicate the phenotype and some features of the disease. Further studies on the mechanisms of bone marrow failure, anemia, and neutropenia should be possible using the SDS BM-Chip.
Conclusions
This study provides evidence that the human BM-Chip is an effective preclinical model of human BM pathophysiology, enabling analysis of human responses to clinically relevant drug pharmacokinetics profiles and radiation dose exposures. The data show that the BM-Chip supports erythroid differentiation as well as myeloid development and mobilization over four weeks of culture while improving the maintenance of CD34+ progenitors compared to traditional culture methods. Additionally, the BM-Chips were able to better recapitulate the toxicity responses of human bone marrow to clinically relevant dose exposures of AZD2811 and 5-FU and showed an improved ability to recover after injury.
BM-Chips derived from patients with Shwachman-Diamond syndrome showed dysfunctions that parallel key hematological abnormalities observed in vivo and led to the discovery of a neutrophil maturation abnormality that exists in a significant subset of SDS patients. Taken together, next-generation in vitro modeling of human bone marrow using Emulate’s microfluidic organ-chips has great potential to facilitate drug development, disease modeling, and translational studies for a range of hematopoietic disorders and toxicities – all while offering a human-specific alternative to animal testing for regulatory assessment.