Organ-Chip Application
Gene Therapy Research & Drug Development
Evaluate the potency and toxicity of gene therapy vectors
YOUR CHALLENGE
Gene therapy holds enormous promise for treating inherited and acquired diseases, but predicting human response is challenging due to complex mechanisms of action, limited clinical data, and a lack of predictive non-clinical models.
A key challenge gene therapy researchers are working to overcome is the development of gene delivery vehicles—including viral and non-viral vectors—that are both efficient and safe. Unfortunately, conventional models routinely fail to predict the potency and toxicity of these vehicles. The limited complexity of conventional in vitro models results in non-physiological response. Meanwhile, the species translation issue for animal models is profound, with two studies showing a ~20-fold higher transduction efficiency in mouse liver cells compared to human. Additionally, the long timelines, high costs, and rigid designs of non-human primate studies limit researchers’ ability to rapidly test and iterate on gene therapy delivery vehicles.
How we can help
Obtain results more likely to translate to human response by assessing gene therapy vectors in a more physiologically relevant microenvironment.
With a variety of Organ-Chip offerings, researchers can evaluate the toxicity and tissue-specific tropisms of both viral and non-viral vectors—a key challenge when developing gene therapy. To model intravenous infusion of gene therapy vectors, the two-channel design of Emulate Organ-Chips can be used to assess gene therapy vector transport from the vasculature into the target tissue, as shown in proof-of-concept studies.
Supported Application
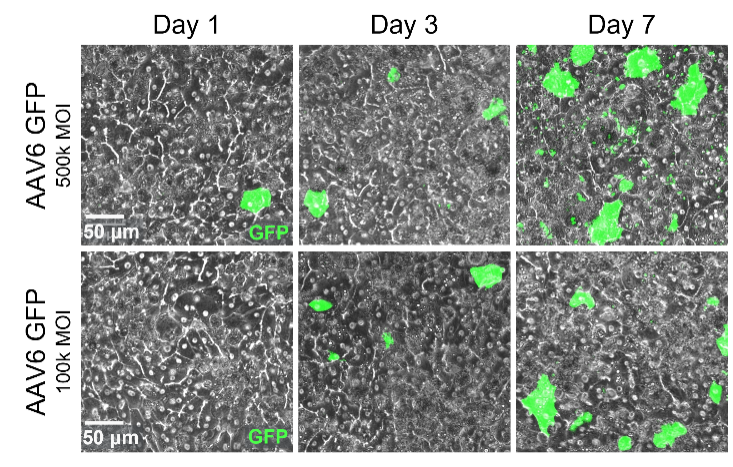
Liver Adeno-Associated Virus (AAV) Transduction
A more human-relevant model for vector testing and optimization
Developing safe and effective viral vectors for gene therapy is time consuming and challenging. Non-human primate studies are slow, costly, and tightly regulated, conventional in vitro models have limited complexity that results in non-physiological response. With the adeno-associated virus transduction application for the Emulate Liver-Chip, you can rapidly test and iterate on AAV design in a human-relevant model of the liver sinusoid to accelerate vector optimization. By evaluating potency and safety in a primary human-based Organ-Chip model, you can avoid species translation issues to improve clinical success.
customer highlight
Assessing Transduction Efficiency of Lipid Nanoparticle-encapsulated mRNA
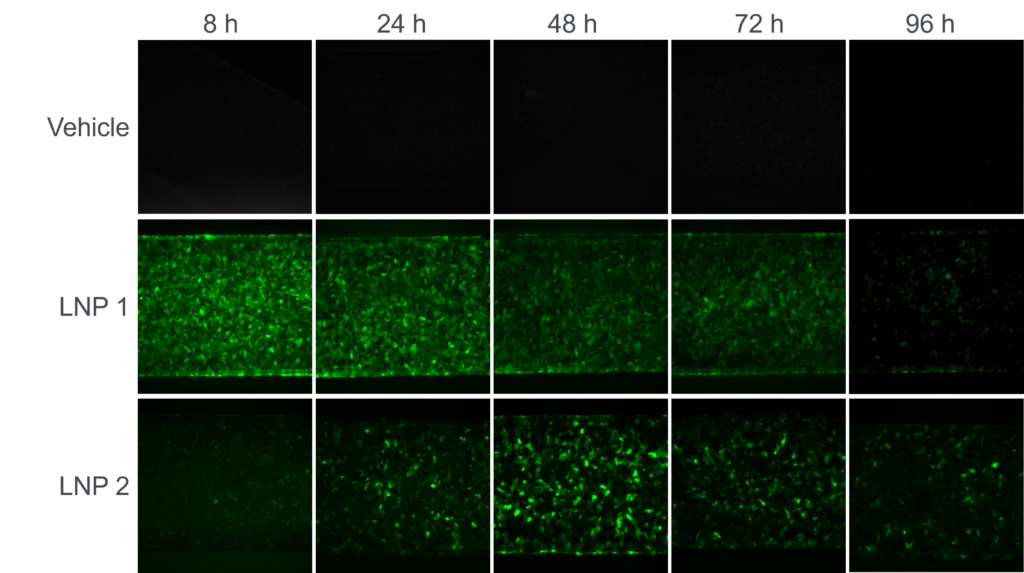
In a service study for a leading global pharmaceutical company, the Emulate Liver-Chip was used to assess the transduction efficiency of lipid nanoparticle (LNP)-encapsulated mRNA. In this study, the team administered two different LNPs containing green fluorescent protein (GFP) mRNA in the epithelial channel and monitored the results over 96 hours.
Results from the Liver-Chip captured the expected difference in expression profile: The first LNP induced GFP expression that started high and gradually tapered off, while the second LNP induced delayed expression that peaked after 48 hours.
This study shows that Emulate Organ-Chips can model time-dependent differences in the transduction efficiency for non-viral vectors and provide human-relevant insights that researchers can use to optimize vector design and improve clinical success.
customer highlight
Screening LNP candidates for toxicity
To increase the efficiency of her research program at Moderna, Dr. Samantha Atkins started using the Emulate human Liver-Chip to screen for LNP-mediated toxicity instead of relying solely on NHPs.
In a simple cost analysis, Dr. Atkins found that she was able to screen 35 novel LNPs in the Liver-Chip during a course of experiments that took 18 months at a total cost of $325,000. If she were to screen the same number of LNPs using traditional NHP studies, it would have cost Moderna over $5,000,000 and taken over 60 months to complete.
Learn more about how Dr. Atkins is using Organ-Chips to improve her research program, and calculate your own cost savings.