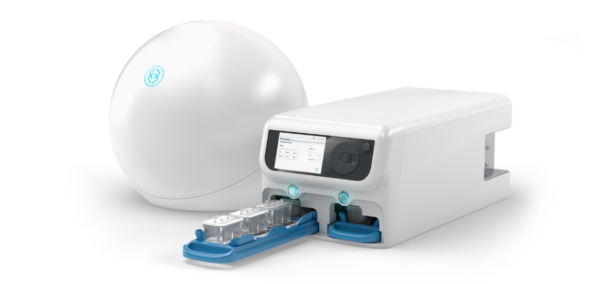
organ-Chip model
Liver-Chip
A new gold standard for assessing human-relevant liver toxicity. Accepted into the FDA’s ISTAND program for prediction of DILI.
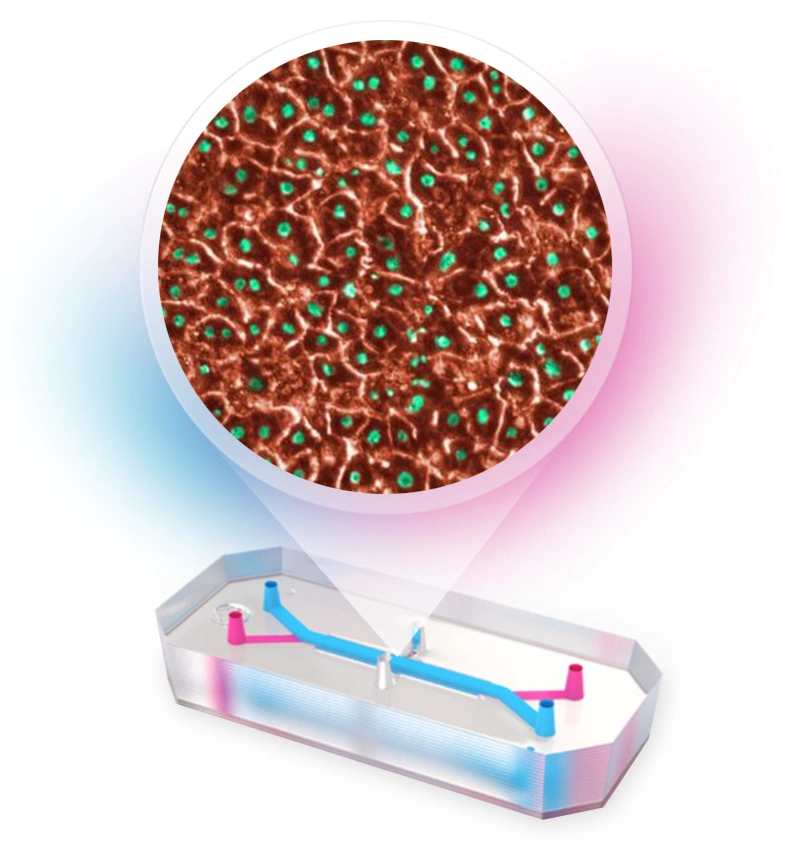

The Liver-Chip is a BioKit Model that Emulate has internally developed and validated. It is available as a BioKit, which includes pre-qualified cells, Organ-Chip consumables, and validated protocols, with guarantees on characterization and functionality.
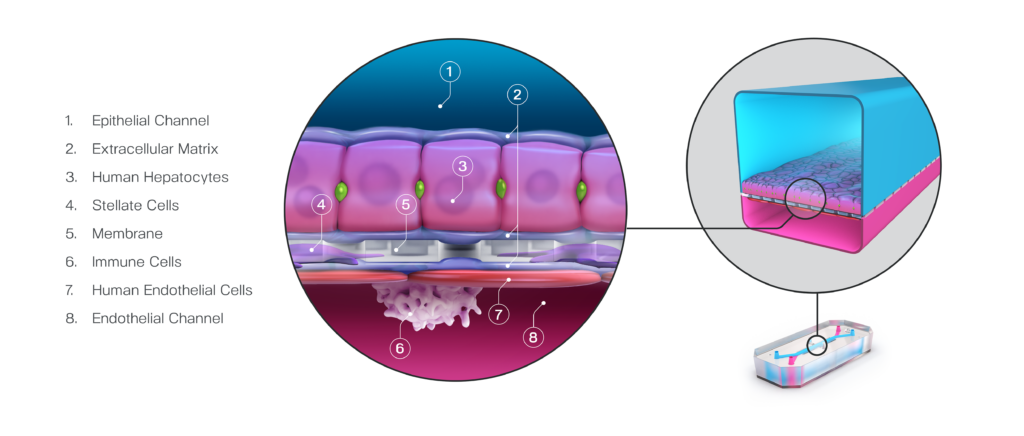
Characterization
A validated and comprehensive preclinical human liver model
The Emulate human Liver-Chip faithfully recapitulates in vivo physiological functions of the human liver through critical microenvironment features such as 3D multicellular architecture and vascular flow. These features enable the Emulate human Liver-Chip to provide a more human-relevant model of the liver compared to conventional sandwich cultures, spheroids, and animal models, each of which lack relevant features to appropriately model the human liver.
Category | Feature | Liver-Chip | Sandwich Cultures | Spheroids | Animal Models |
Human Relevance | Human Cells | Yes | Yes | Yes | No |
Physiologically Relevant | +++ | + | + | ++ | |
Accurate Prediction of | +++ | - | ++ | + | |
Microenvironment | Multi-cellular Complexity | ++ | + | + | +++ |
Vascular / Media Flow | Yes | No | No | Yes | |
Tissue-specific ECM | Yes | No | No | Yes | |
Experimental Design | Tunable Dosing Parameters | ++ | ++ | ++ | + |
Duration of Experiment | ++ | - | ++ | +++ | |
Clinically Relevant Assays | +++ | + | + | ++ | |
Convenience | Speed to Insight | ++ | +++ | +++ | + |
Ease of Learning Curve | ++ | +++ | +++ | + | |
Cost-effectiveness | ++ | +++ | +++ | + | |
Characterization
A validated and comprehensive preclinical human liver model
The human Liver-Chip faithfully recapitulates in vivo physiological functions of the human liver through critical microenvironment features such as 3D multicellular architecture and vascular flow. These features enable the Liver-Chip to provide a more human-relevant model of the liver compared to conventional sandwich cultures, spheroids, and animal models, each of which lack relevant features to appropriately model the human liver.
Category | Human Relevance |
Feature | Human cells |
Liver-Chip | Yes |
Sandwich Cultures | Yes |
Spheroids | Yes |
Animal Models | No |
Category | Human Relevance |
Feature | Physiologically relevant |
Liver-Chip | +++ |
Sandwich Cultures | + |
Spheroids | + |
Animal Models | ++ |
Category | Human Relevance |
Feature | Accurate prediction of |
Liver-Chip | +++ |
Sandwich Cultures | - |
Spheroids | ++ |
Animal Models | + |
Category | Microenvironment Complexity |
Feature | Multi-cellular complexity |
Liver-Chip | ++ |
Sandwich Cultures | + |
Spheroids | + |
Animal Models | +++ |
Category | Microenvironment Complexity |
Feature | Vascular/media flow |
Liver-Chip | Yes |
Sandwich Cultures | No |
Spheroids | No |
Animal Models | Yes |
Category | Microenvironment Complexity |
Feature | Tissue-specific ECM |
Liver-Chip | Yes |
Sandwich Cultures | No |
Spheroids | No |
Animal Models | Yes |
Category | Experimental Design |
Feature | Tunable Dosing Parameters |
Liver-Chip | ++ |
Sandwich Cultures | ++ |
Spheroids | ++ |
Animal Models | + |
Category | Experimental Design |
Feature | Duration of Experiment |
Liver-Chip | ++ |
Sandwich Cultures | - |
Spheroids | ++ |
Animal Models | +++ |
Category | Experimental Design |
Feature | Clinically Relevant Assays |
Liver-Chip | +++ |
Sandwich Cultures | + |
Spheroids | + |
Animal Models | ++ |
Category | Convenience |
Feature | Speed to Insight |
Liver-Chip | ++ |
Sandwich Cultures | +++ |
Spheroids | +++ |
Animal Models | + |
Category | Convenience |
Feature | Ease of Learning Curve |
Liver-Chip | ++ |
Sandwich Cultures | +++ |
Spheroids | +++ |
Animal Models | + |
Category | Convenience |
Feature | Cost-effectiveness |
Liver-Chip | ++ |
Sandwich Cultures | +++ |
Spheroids | +++ |
Animal Models | + |
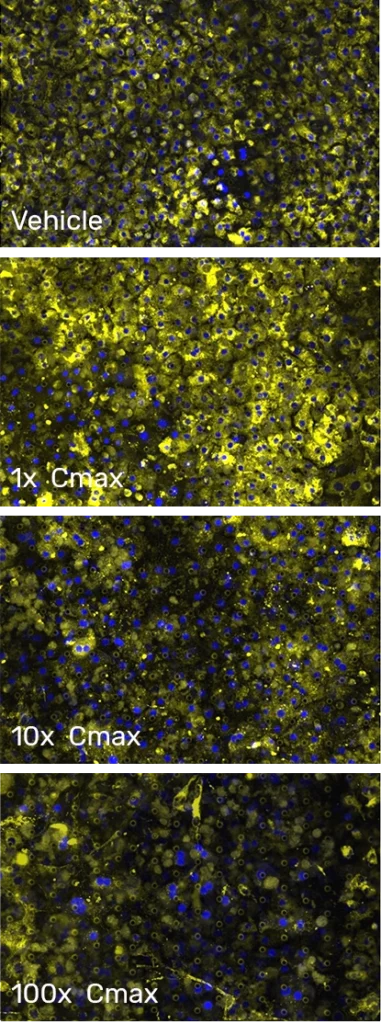
Hepatotoxicity
RESEARCH CHALLENGE
Drug-induced liver injury remains a primary cause of drug candidate attrition and withdrawal from the market
Current preclinical models inadequately predict hepatotoxicity and DILI in humans. Poor clinical translation arises from species differences present in animal models and the limited in vivo-relevant physiology of conventional liver cell culture. This not only leads to high preclinical and clinical attrition, but it can also pose serious health risks to patients.
Liver-Chip Benefits
A more predictive in vitro model of human hepatotoxicity
In a landmark study in Communications Medicine, part of Nature Portfolio, researchers demonstrated that the Emulate human Liver-Chip could improve patient safety and reduce small-molecule clinical trial failures due to liver toxicity by up to 87%, with 100% specificity. The Emulate human Liver-Chip enables researchers to assess diverse mechanisms of toxicity with greater fidelity than animal testing or conventional cell culture, lowering the risk of DILI and clinical trial attrition for drug candidates.
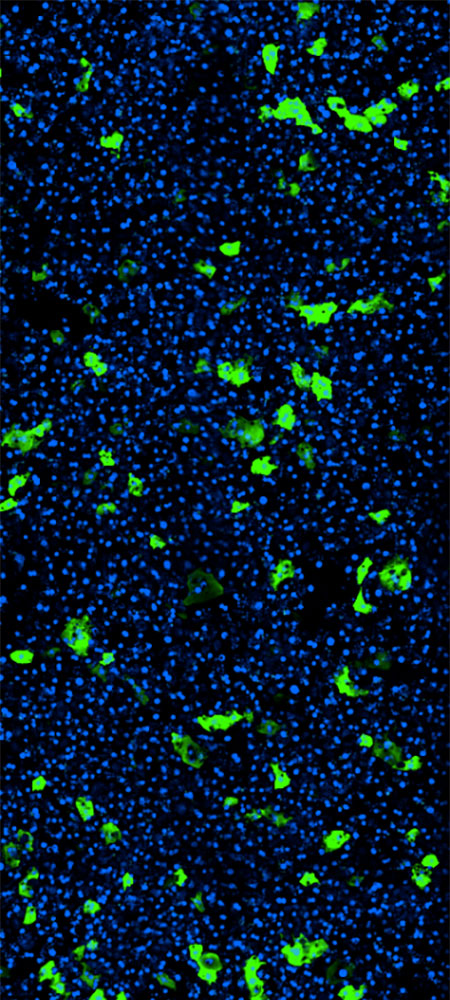
AAV Transduction
RESEARCH CHALLENGE
Development of safe and efficient gene therapy vectors remains slow
Gene therapy holds enormous promise for inherited and acquired genetic diseases, yet progress remains slow. Developing safe and effective viral vectors is time consuming and challenging. Animal studies are slow, costly, and tightly regulated, while conventional in vitro models’ limited cellular complexity results in non-physiological response. These challenges have led not only to a slow pace of gene therapy approval, but also serious safety risks to patients.
Liver-Chip Benefits
A faster path to AAV optimization
With the Emulate human Liver-Chip, researchers can test the delivery efficiency and safety of adeno-associated virus (AAV) vectors in a human-relevant model of the liver sinusoid and get results in weeks—not months, like with animal models. With this application, it is possible to rapidly iterate on AAV design and explore each cell type’s contribution, accelerating optimization ahead of clinical trials.
Compatible Platforms
Get Started Today
Experience the predictive power of Organ-on-a-Chip technology. The Liver-Chip can be created with either a BioKit or a Basic Research Kit.
FEATURED RESOURCE
Liver Toxicology White Paper
This white paper outlines the benefits of incorporating the Liver-Chip S1 into preclinical workflows and its potential to reduce costs and bring safer drugs to market faster. It also introduces the Liver-Chip R1, a new liver model designed to improve testing accuracy for lipophilic drugs by reducing drug absorption, further enhancing predictive toxicology study precision.