Gene therapies have generated both excitement and hope, as they have the potential to correct underlying genetic defects and cure diseases. However, one of the major hurdles gene therapy researchers face is delivering enough molecular material into target cells to make a difference—a key aspect of this type of treatment.
Many labs have turned to adeno-associated viruses (AAVs), which can be excellent vehicles for delivering genetic material and/or editing tools. They persist in host cells and present low immunogenicity and toxicity risks. In addition, AAVs are easily modifiable, stable, and adaptable to many tissues. However, the same labs have faced difficulty when researching AAVs using conventional models, such as 2D cell cultures and animals.
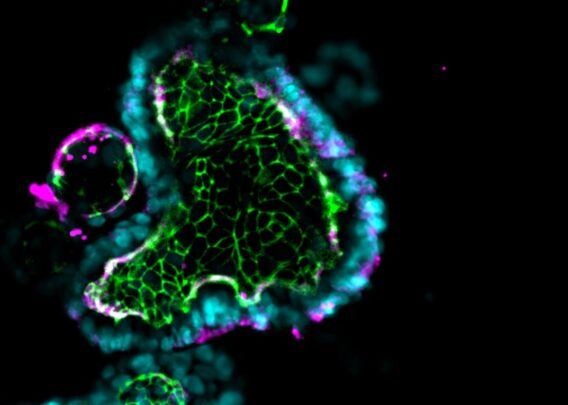
The Emulate Liver-Chip offers a more human-relevant way of testing viral vectors during early development and preclinical studies compared to conventional models. The Liver-Chip incorporates multiple cell types in a dynamic microenvironment, allowing researchers to assess virus-mediated transduction efficiency and toxicity to develop safe, effective treatments.
BARRIERS TO GENE THERAPY
AAVs have been used in therapies for hemophilia, inherited liver diseases, neurological conditions, and other disorders. While there are only a few gene therapies in the clinic, there are many on the horizon.
But the distance to that horizon has become problematic, as AAV-based therapy development has been slow and costly. Since labs lack good pre-clinical models to robustly study toxicity and gene delivery efficacy, they are being forced to take the long road.
A major concern for any emerging gene therapy is hepatotoxicity risk, as even the most precisely targeted vectors often accumulate in the liver. In fact, hepatotoxicity is the most common stumbling block seen in clinical trials for AAV therapeutics.
Current pre-clinical studies largely use 2D cell cultures and animal models. In vitro cell models provide inadequate data because they lack the complexity to capture in vivo physiology. These 2D models generally have one cell type—often hepatocytes for liver studies—but do not incorporate fluid flow and other biomechanical forces found in vivo. In addition, non-human primate studies are slow, costly, and tightly regulated, limiting the pace of viral vector optimization.
The life sciences industry needs advanced technologies that accurately mimic human physiology to effectively translate pre-clinical results into successful human trials. Because Organ-on-a-Chip technology more closely recapitulates in vivo functions, it predicts human responses far more reliably than other models. Organ-Chips accurately replicate human physiology by including dynamic media flow, cellular complexity, 3D cellular architecture, and cell-cell signaling.
By providing a more human-relevant in vitro platform for AAV-based therapeutic development, Organ-Chips can help researchers rapidly iterate on AAV designs to accelerate gene therapy development, reduce clinical trial attrition, and bring new products to patients faster.
THE EMULATE LIVER-CHIP IN ACTION

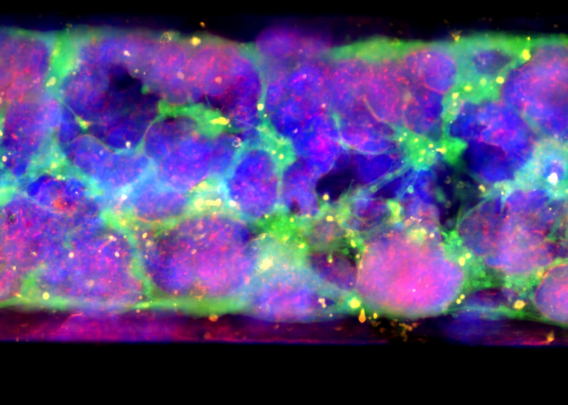
The Liver-Chip co-cultures primary human hepatocytes and liver sinusoidal endothelial cells under tissue-specific media flow. It also enables the addition of Kupffer and stellate cells, allowing researchers to capture additional cell-cell interactions. As a result, it better represents human in vivo liver tissue than conventional approaches and facilitates measurements of AAV transduction efficiency and toxicity differences across space, time, and cell types.
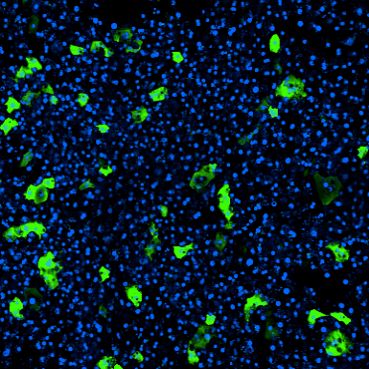
To assess the Emulate Liver-Chip’s ability to predict AAV transduction efficiency and hepatotoxicity risk, chips were treated with six conditions—including multiple MOIs (multiciplicities of infeciton) of AAV2 and AAV6 vectors—administered for 24 hours via the epithelial channel. After treatment, they were monitored and measured for transduction efficiency and safety.
During the seven-day monitoring period, the chips displayed concentration-, time-, and serotype-dependent transduction. Researchers also assessed morphology and functional liver endpoints (albumin, ALT) and confirmed that there were no toxic responses, showing how the model can be used to assess AAV serotypes’ potential to induce toxicity in human liver tissue.
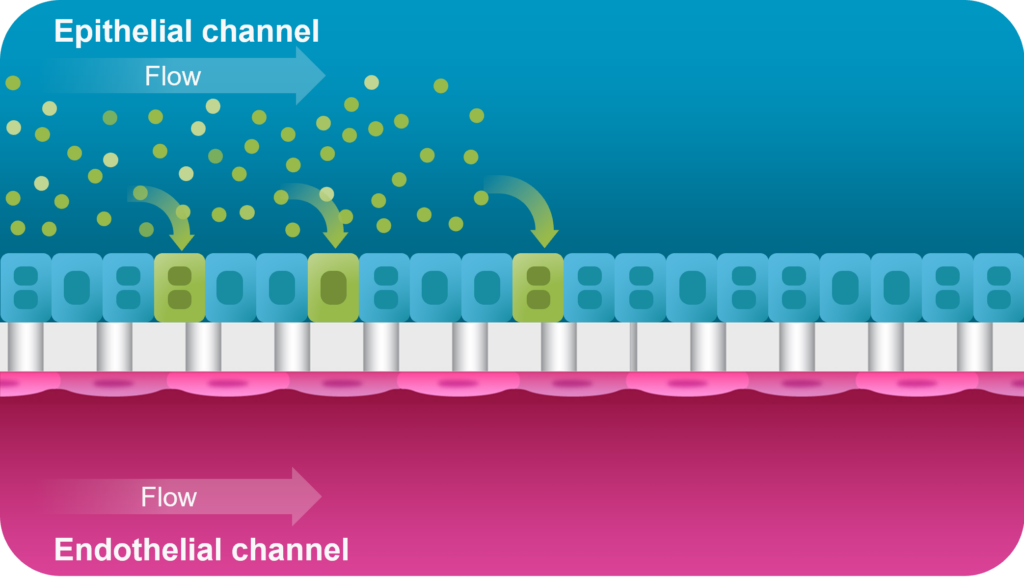
A proof-of-concept study showed that the Emulate Liver-Chip can model AAV vector transport across vascular tissue for subsequent transduction of epithelial tissue, allowing researchers to emulate the intravenous administration route for many AAV-based gene therapies. Furthermore, AAV was administered via the vascular channel and demonstrated successful hepatocyte transduction.
The Emulate Liver-Chip successfully demonstrated its potential to accelerate workflows for AAV gene therapy development and preclinical testing. With this important tool, researchers can accumulate actionable data in weeks, rather than the months these studies might take in animal models. Since the Liver-Chip facilitates multiple rapid iterations—which is critical to designing effective gene therapies—scientists can gain deeper insights into AAV design to bring safe, effective treatments—even potential cures—to patients.