As many as 90 percent of new drug compounds fall short in clinical trials. Many of them fail because the compounds are toxic in humans, particularly to the liver and kidneys. Though pharmaceutical companies use cell cultures and animal models to assess toxicity issues before their drugs go to human trials, those approaches have shown mixed success.
This established preclinical workflow is wasteful on many levels. Drug companies expend precious resources on new drug applications that are doomed to failure. Clinical trial participants are often exposed to toxic compounds, and some have paid dearly. Patients hoping for more effective medicines must continue to wait.
In an ideal world, pharma companies would fully understand a drug’s potential toxicities long before it goes to human trials. If the compound is found to have toxicity, medicinal chemists could alter the molecule to mitigate the problem. In other cases, resources could be shifted to drugs that have fewer toxic effects.
To get there, we need to augment the preclinical workflow, leverage new models, and provide better data on drug toxicity with in vitro toxicology testing at the early stages of research and development. Organ-Chips are a promising option.
The Advantages of Organ-Chips
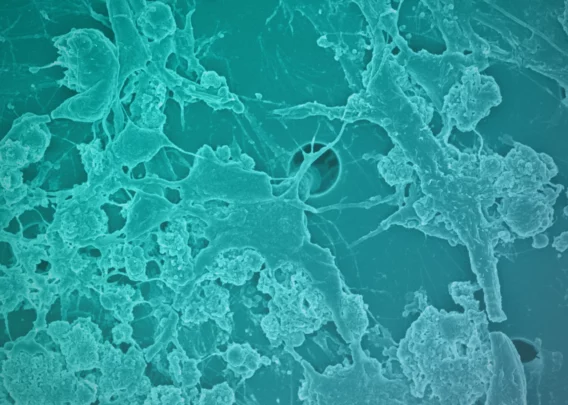
Emulate has developed liver and kidney chips that can provide truer readouts on drug toxicity. These small microfluidic chips, about the size of an AA battery, are designed to better replicate the forces and signals cells encounter in vivo.
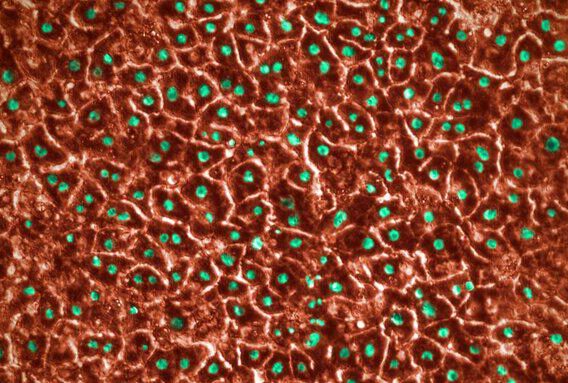
Kidney toxicity is a major reason therapeutic compounds fail in human trials. Toxicity often shows up in the renal proximal tubules, which are responsible for filtering the blood. The Emulate human Proximal Tubule Kidney-Chip was created to model this behavior. The chip contains both tubular and vascular endothelium to replicate mechanical and physiological forces in the body, such as cellular signaling and shear stress, and provide a model that will more accurately predict how these cells respond to medicines or other compounds.
In addition, because organ-chips are made from a clear polymer, they can be visualized. For example, researchers can easily examine cellular morphology under a microscope.
Emulate recently tested the Kidney-Chip against cisplatin and gentamycin, which are known nephrotoxic agents. The cells in the chip reacted to these toxins much like cells in the body, showing obvious injuries. In addition, by studying gene expression and other factors, Emulate researchers showed the chips reproduced cellular transporters, which play a significant role in toxicity, and other important kidney mechanisms. Ultimately, the study showed the chip offers a physiologically relevant way to assess drug safety.
A Dynamic Preclinical Model
Instead of studying compounds on cell cultures, followed by animal models, followed by human participants, researchers can layer in this new model, which more closely replicates how cells behave in the body. Organ-Chips will not replace cell culture or animal models in the immediate future. Rather, they provide a vehicle to inform preclinical data and ultimately predict a drug’s potential toxicity.
The goal is to catch toxicity much earlier in the process, a refinement that could reduce waste, giving pharma companies better data to decide which compounds move forward into new drug applications and which ones should be reexamined.
Having this extra layer of data could ultimately make clinical trials more efficient, increasing success rates, reducing the dangers to trial participants and accelerating the drug discovery process.