For the better part of the last century, much biological research was built upon in vitro experiments—commonly, cells growing in a dish—with the assumption that results from these cultures may translate into insights that are relevant to humans. Because in vitro studies generally lack three-dimensional (3D) context, mechanical forces, cellular microenvironment, and multi‐organ physiology of whole organisms, many researchers are starting to question the clinical relevance of findings obtained with two-dimensional (2D) in vitro models.
Compounding this issue is that the physiological relevance of 2D in vitro results must be validated using animal studies. However, using animals in research is controversial, with evidence suggesting that data are often not of significant benefit to humans, and raises ethical concerns about animal welfare. Biopharmaceutical companies around the world are looking to reduce, refine and eventually replace animal models for preclinical drug development, toxicology studies and disease modeling to save time, reduce costs and be more predictive for clinical trials.
2D vs 3D Cell Culture: More Refined In Vitro Models
Cell culture technology overall has been slow to innovate, and progress has been incremental over the past 50 years, with several notable exceptions for challenging cell types and more complex experimental systems. Transwells commonly consist of compartments housing several cell types and/or soluble factors that are separated by one or more selective membranes. Incorporation of extracellular matrix, such as Matrigel, enables cells such as tissue-derived or pluripotent stem cells to grow in culture. The use of pluripotent stem cells, which can be coaxed into developing different lineages, has helped researchers work within more accurate models. Spheroid culture technology allows cells to grow together in suspension, recreating gradients within a three-dimensional structure. Further refinements create multicellular niches that replicate organoid structures. These experimental systems are often best suited for defined processes such as high-throughput screening, dosage studies, or cellular responses at the gene or protein level. Most also require specialty cell culture media and supplemental factors, as well as manual cell passaging steps that pose risks for contamination.
Stay Up to Date with the Emulate newsletter
A More “Natural” 3D Cell Culture Model
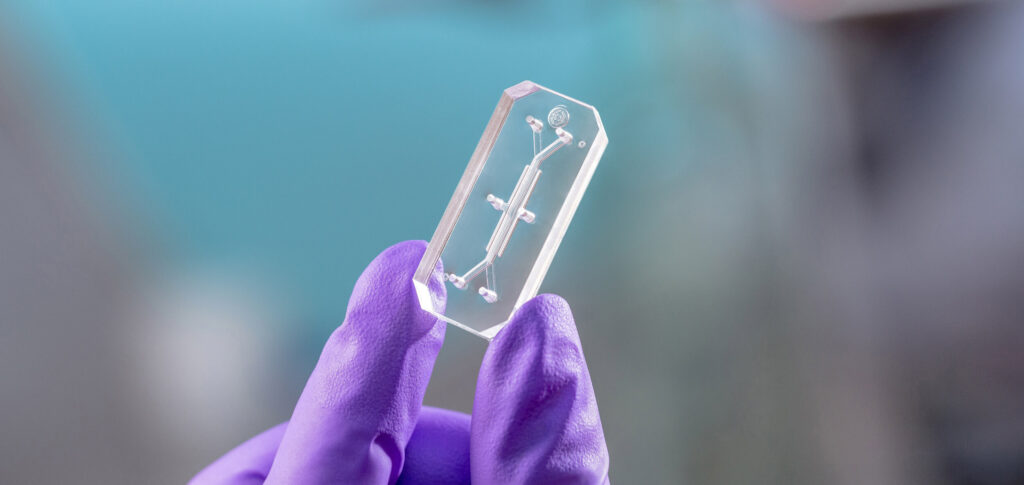
Enter Emulate, poised to offset biopharma’s reliance on animal models and traditional cell culture with Organs-on-Chips (OOC) technology. Organ-Chips are engineered microphysiological systems (MPS)—dynamic models with defined composition at the cell, tissue, and organ level—allowing discovery of contributors to human physiology and pathophysiology. Organ-Chips offer an advantage over many MPS and organoid culture systems because they rely on microfluidics to mimic vascular perfusion, tissue–tissue interfaces, and other important mechanical cues, such as stretch to emulate breathing or intestinal peristalsis. As “living 3D cross-sections of the functional units of key organs,” experiments with Organ-Chips must be carefully designed around the right experimental questions and relevant endpoints. Researchers can also layer on complexity to these models in the form of soluble factors and different cell types (e.g., iPSC, epithelial, endothelial, connective tissue, immune, nerve, muscle, commensal or pathogenic microbes) to expand their models, which is usually not possible with animal studies.
Organ-Chips with different fluidic designs can be used with primary, patient‐derived or differentiated cells to gain insight into human‐relevant cellular and molecular mechanisms of pathogenesis, as well as drug response. These robust models can be probed using a wide array of analytical techniques including high resolution microscopy, flow cytometry, genomics, proteomics, metabolomics, and histological analysis. With future development of integrated electrical, chemical, mechanical, and optical sensors, real-time readouts of tissue barrier integrity, electrical activity, oxygen utilization, pH, and molecular transport could be obtained. Emulate Organ-Chips were even sent to the International Space Station for experiments on the physiology of microgravity.
As Organs-on-Chips technology becomes a better replicate of living beings, more questions arise to define the parameters that need to be reproduced. Emulate OOC technology is being used by researchers around the world to better understand COVID-19 disease processes and to play a role in the development of new therapeutics. Whether on the earth or flying high above it, OOCs are setting a new standard for predicting how humans respond to medicines, chemicals, and foods with greater precision and control than conventional cell culture or animal-based testing methods.
Cited:
“Special Report on Animal Models: All In” Randall C. Willis, DDN News July/Aug 2020.
“Is it Time for Reviewer 3 to Request Human Organ Chip Experiments Instead of Animal Validation Studies?” Don Ingber, founding director of the Wyss Institute & Chair of Emulate Scientific Advisory Board. Advanced Science Oct 12, 2020.