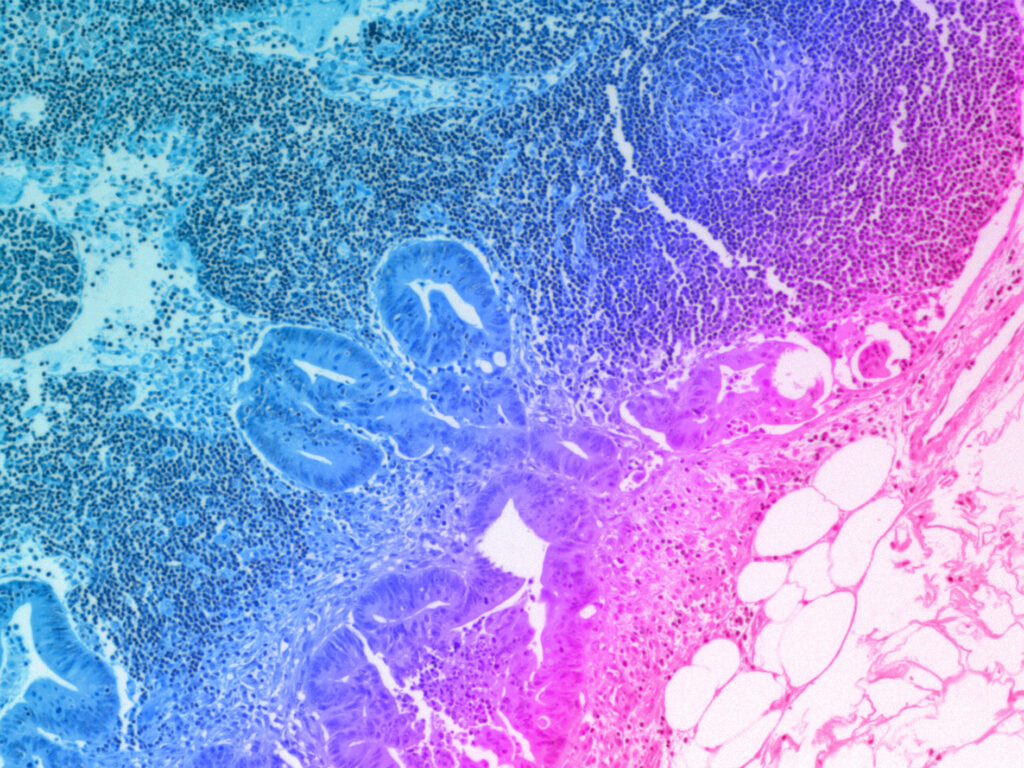
Testing new vaccines against seasonally emerging influenza strains is a necessary process, due to frequent changes in the virus’s immunogenic surface proteins. Vaccines and other drugs, such as immunotherapies, are evaluated for efficacy and safety in vitro using cultured human immune cells and in animal models. However, these assessments often fail to fully predict human responses, because they lack the biologically relevant microenvironment in which to develop an adaptive immune response.
Modeling a true human immune response is a complex undertaking. Primary lymphoid organs, like the thymus and bone marrow, allow immune stem cells to proliferate, differentiate, and mature. Secondary lymphoid organs like the lymph nodes, spleen, and tonsils maintain mature but naïve cells ready to be activated by contact with antigen presenting cells, leading to clonal expansion and affinity maturation for the adaptive immune response. Lymphoid follicles (LFs) that are present within lymph nodes or form ectopically within other secondary lymphoid organs contain germinal centers that support the development of plasma cells that secrete high affinity antibodies.
A human model of the LF would provide a better understanding as to why these organ structures are critical for developing adaptive immune responses and would be extremely useful for preclinical assessment of vaccine efficacy. This study represents the first-of-its-kind human LF-Chip containing B, T, plasma and antigen-presenting cells to test functional immunization responses to vaccines and adjuvants in vitro.
- Research Area: Vaccine and adjuvant testing
- Organisms: Human
- Sample Types: Peripheral blood-derived immune cells
- Research Question: Can the LF-Chip be a useful human-relevant preclinical tool for assessing the efficacy and safety of influenza vaccines and adjuvants?
Experimental Overview
Characterizing the LF-Chip
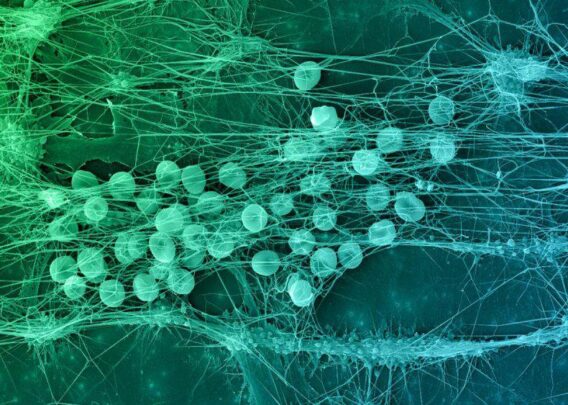
Researchers created the LF Organ-Chip by using peripheral blood mononuclear cells (PBMC) isolated from donor blood and culturing the cells at a high density within the extracellular matrix (ECM) in the lower chamber of the chip, with culture medium flow supplied in the upper chamber. The researchers observed self-aggregation of lymphocytes within the ECM resembling which resembled germinal centers. They did not see autoactivation of B cells (determined by levels of IgG or IgM in the Organ-Chip cultures), overcoming a key challenge seen in static 2D cultures.
Within the LF-like structures formed in the chip, the researchers detected molecules that indicate immune reaction, including the chemokine CXCL13, which recruits T cells, and activation-induced cytidine deaminase (AID) expression, which is required for the critical antibody class switching step. Class switching and plasma cell formation were observed, and, when exposed to an antigen that mimics the presence of dead bacteria, a robust IgG response was generated, suggesting that the model faithfully recapitulated a functional adaptive immune response.
Vaccination Testing on the LF-Chip
Protective immunity induced by vaccination requires plasma cell production of antigen-specific antibodies. To see if the model could simulate an immune response to vaccination with viral antigens with or without an immune stimulating adjuvant, the authors added patient monocyte-derived dendritic cells in a 3D ECM gel within the Organ-Chip and tested it with influenza virions and adjuvant. The germinal centers showed plasma cell formation, expression of AID by resident B cells, and production of cytokines, and robust production of antibodies to influenza virus hemagglutinin – similar to vaccinated humans. Notably, these results were not replicated within a 2D static cell culture model. A commercial influenza vaccine was used to induce a clinically relevant immune response in the chip, showing important features like cytokine response and donor variability.
Towards a Better Preclinical Assessment of Vaccines and Adjuvants
This is the first reported in vitro model that supports formation of human LFs with functional germinal centers similar to those found in secondary lymphoid organs in vivo using cells isolated from peripheral blood. Within the LF-Chip, B and T cells support plasma cell differentiation and antibody secretion while spontaneously forming LFs that express the correct cytokines and chemokines. These Organ-Chip microphysiological systems are also patient-specific and can recapitulate donor variability in response to vaccination.
Previous attempts at recreating cell culture models of the lymph node for preclinical vaccine testing are not as useful for modeling the human adaptive immune response. These experimental models do not result in the appropriate biomarkers, de novo lymphoid follicle formation, or survival of plasma cells. In addition to ethical concerns and shortages of non-human primates due to the current COVID-19 pandemic, animal models do not usually replicate an intact human immune response. Taken together, these findings suggest that the LF-Chip may be a useful human-relevant tool for assessing the efficacy and safety of vaccines and adjuvants in a patient-specific manner.
Future Uses for the LF-Chip
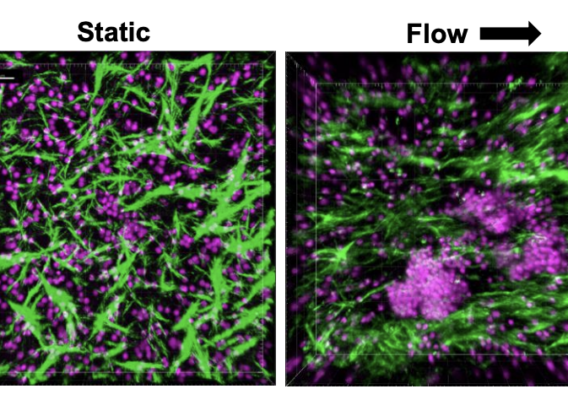
Using primary human blood cells collected non-invasively, the LF-Chip can also be used to study the basic biology of immune organ formation as well as antigen-induced immunological responses. Specifically, results obtained in this study suggest that the presence of dynamic fluid flow promotes LF formation and reduces autoactivation of B cells, possibly facilitated by the ECM to promote proper cell interactions. Future work with the chip could explore how mechanical forces work to support organ formation at the cellular level. The LF-Chip may also be used to understand control of genes encoding cytokines and chemokines that govern the microenvironment critical for formation of germinal centers and immune cell activation. Using high-resolution imaging, many of the cellular and molecular processes occurring in the LF-Chip can be observed over time, enabling valuable longitudinal studies of human immune responses.