In April 2021, a bipartisan congressional committee took a big step towards modernizing the way therapeutics are developed in the United States by proposing the FDA Modernization Act of 2021—an amendment to the 1938 Federal Food Drug and Cosmetics Act (FFDCA). Among other stipulations, the FFDCA currently mandates the use of animal testing during preclinical drug development. The FDA Modernization Act of 2021 aims to change this by broadening the scope of acceptable preclinical models for drug development, enabling researchers to test a drug’s safety and efficacy using more advanced and humane methods in place of animal testing wherever possible. Such an amendment, if enacted, could pave the way for faster, more effective, and more humane therapeutic development.
Emulate is proud to endorse the FDA Modernization Act of 2021. Here’s why:
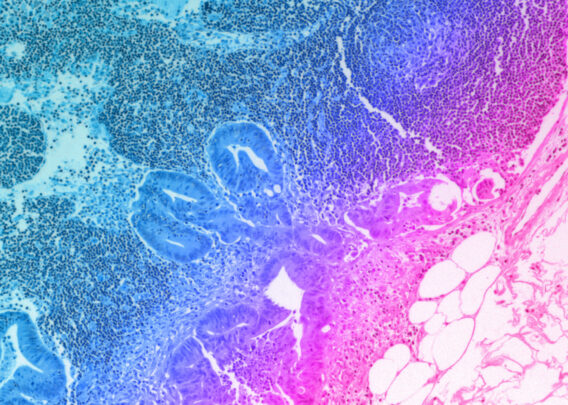
Better models lead to better therapeutics
In its current state, the FFDCA stipulates that drugs can only advance to clinical trials if there is enough data describing the drug’s chemistry, manufacturing, and “primary data tabulations from animal or human studies.” The goal of this stipulation is to ensure that drugs are well characterized from both a chemical and biological perspective before they can be used for clinical trials.
When the FFDCA was enacted in 1938, animal models seemed like the best way for drug developers to study how therapeutics might act within a biological setting. In theory, then, animal testing should ensure that only the safest, most effective drugs make it to clinical trials. However, over the years, evidence has accumulated suggesting that this isn’t always true and that better models are needed.
Some studies estimate that as much as 89% of novel drugs entering clinical trials ultimately fail, with as much as 50% of those failing due to toxicities unanticipated by animal models.
Take, for instance, the dismal failure rate among cancer therapeutics that initially show promise in animal trials, but only about 8% of which successfully progress through clinical trials. Some studies estimate that as much as 89% of novel drugs entering clinical trials ultimately fail, with as much as 50% of those failing due to toxicities unanticipated by animal models.
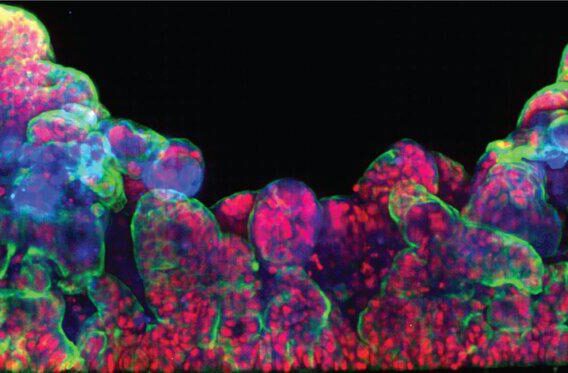
The FDA Modernization Act of 2021 opens the door for researchers to use the best models available to them. With the ability to use better model systems — like Organs-on-Chips — it is likely that candidate drug success rates will increase.
Removing mandates for animal testing enables fast, efficient, and humane drug development
Industry reports estimate that it takes 10 to 15 years for a drug to go from initial hit discovery to clearing clinical trials. Any step we can take to shorten this timeframe without sacrificing drug quality is paramount.
One area for improvement is in preclinical testing. Typically, drug development begins with a large library of potential candidate therapeutics. Initial screening identifies candidates that are most likely to succeed, and these progress through iterative cycles of adjusting the chemical structure and screening for improved properties. Eventually, multiple candidates are ready to progress into more complex systems to analyze efficacy and safety in a biological setting.
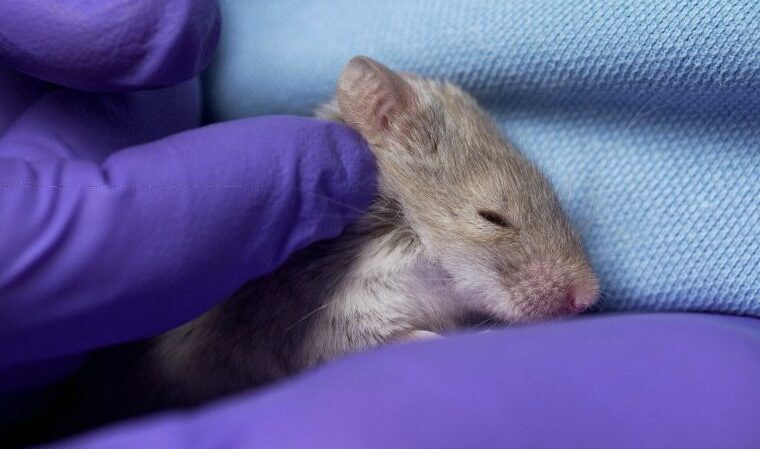
Today, this often means using animal studies. Replacing animal studies with more advanced, human-relevant models has significant potential to improve decision-making throughout the drug discovery and development lifecycle as well as decrease the number of adverse effects that occur in clinical trials.
Additionally, working with animal models can be very resource intensive, as the animals require adequate housing, food, and care. In contrast, models like Organs-on-Chips are much more compact and require less oversight. Estimates suggest that, relative to in vitro models, animal testing is 1.5- to 30-times as expensive. Therefore, removing the need for animal testing is likely to significantly decrease the cost of drug development, which today can exceed $1 billion.
Finally, with the existence of advanced cell-based and microphysiological systems, the FFDCA’s stipulation that animal testing is necessary for preclinical safety and efficacy studies is outdated and inhumane. While animals have a profound and critical role in many aspects of scientific research, the using animals in place of more effective models is both counterproductive and needlessly cavalier about the animals’ welfare.
The FDA Modernization Act of 2021, consistent with the FDA’s initiative to advance regulatory science, will promote animal welfare without compromising scientific research and will ensure that we are better prepared for future challenges and opportunities. At Emulate, we believe that using the most predictive technologies available will ultimately streamline drug development and spur innovation, benefiting both patients and the pharmaceutical industry.