In 1817, Dr. James Parkinson wrote about an enigmatic disease affecting his patients, a neurodegenerative condition characterized by the progressive decline of motor skills and an “involuntary tremulous motion.” Parkinson had no way of knowing that this condition, now known as Parkinson’s disease, was the result of dopamine-producing neurons dying off in large parts of patients’ brains, specifically within a region known as the substantia nigra. Though more than 200 years of research separate us from Parkinson’s first descriptions, many aspects of Parkinson’s disease still remain a mystery.
One such mystery revolves around a protein known as ɑ-synuclein. Observations suggest an abnormal buildup of ɑ-synuclein protein bundles in neuronal tissues which causes inflammatory signaling, mitochondrial dysfunction, and worsening disease. This protein’s prominence in patients with Parkinson’s disease leads many experts to believe that ɑ-synuclein bundles may drive disease pathology; however, the mechanism remains unknown.
To gain a deeper understanding of Parkinson’s disease pathology and the potential role of ɑ-synuclein, researchers need powerful disease models that allow them to observe how various cell types within the substantia nigra behave in the presence of ɑ-synuclein bundles. In work supported in part by the Michael J. Fox Foundation for Parkinson’s Research, Emulate partnered with investigators at Harvard, Northeastern University, and the University of Athens to develop a dynamic “brain-on-a-chip” model that integrates multiple cell types and microenvironmental features to create a system that closely resembles the human substantia nigra. Such a model could be applied to study mechanisms of Parkinson’s disease. The results of these efforts have recently been published in Nature Communications.
Experimental Overview
- Research Area: Disease pathology
- Organisms: Human
- Sample Types: Brain-Chip
- Research Question: Can the Brain-Chip be applied as a more physiologically relevant model of the human substantia nigra to model mechanisms of Parkinson’s disease?
- Results:
- Brain-Chip demonstrated transcriptomic and phenotypic characteristics that more closely resembled in vivo measurements relative to conventional cell culture.
- Perfusion of chips with ɑ-synuclein fibrils resulted in inflammatory signaling, mitochondrial stress, and cell death.
- ɑ-synuclein bundles also negatively affected endothelial tight junctions, resulting in increased permeability which could be attenuated in the presence of Trehalose, an autophagy-inducing therapeutic.
- Conclusion: Brain-Chip more accurately replicates in vivo conditions of the human substantia nigra relative to conventional cell culture and thus presents an accessible and promising model for the study of Parkinson’s disease.
Modeling the human neurovascular unit
Animal models have provided valuable insights into Parkinson’s disease pathology, but their ability to predict human response is limited due to species differences—not least of which being that humans are the only animal known to develop Parkinson’s disease. Conventional cell culture models similarly provide valuable insight into the molecular mechanisms of disease pathology but fail to adequately replicate the complex milieu of the human substantia nigra, which can influence how cells respond to stressors—like ɑ-synuclein bundles—as well as potential therapeutics.
The brain-on-a-chip model presents a promising alternative. Developed by Emulate, the Brain-Chip is a three-dimensional, dynamic system that co-cultures neurons, astrocytes, microglia, pericytes, and microvascular brain endothelial cells under fluid flow conditions and in the presence of tissue-specific extracellular matrix proteins (collagen type IV, fibronectin, and laminin). Cells are selectively seeded into either one of two channels to form a vascular chamber consisting of microvascular endothelial cells and pericytes and a brain chamber containing the neuronal and immune cell types. A thin, porous membrane separates the two chambers, enabling close communication between cells while maintaining distinct environments.
In the present study, dopaminergic neurons were used to model the substantia nigra, the brain region most relevant to Parkinson’s research. A battery of characterization assays confirmed that these chips closely resembled the in vivo substantia nigra neurovascular environment.
A comparison of transcriptomic profiles in the substantia nigra Brain-Chip to those found in both conventional cell cultures (containing the same mixture of cells) and adult human substantia nigra tissue demonstrated that the Brain-Chip more closely resembled in vivo gene expression. Differential gene expression analysis suggested that Brain-Chips encouraged more in vivo-like expression profiles by enhancing cellular maturation.
Modeling Parkinson’s disease on a chip
Having established the substantia nigra Brain-Chip as a valid model, the team proceeded to test if it could replicate aspects of Parkinson’s disease by perfusing the brain chamber with purified ɑ-synuclein bundles.
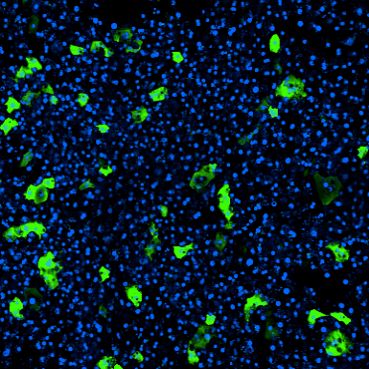
Analyses following continuous perfusion with ɑ-synuclein bundles revealed robust pathological phenotypes: Neurons demonstrated increased cleaved caspase staining (indicative of apoptosis), microglia clustered around dying neurons, and astrocytes showed signs of increased astrogliosis—a hallmark of neuronal inflammation. Α-synuclein bundles also appeared to induce a significant increase in reactive oxygen species in these cells, indicating likely mitochondrial dysfunction. Finally, cells were positive for phosphorylated ɑ-synuclein, which is typically associated with ɑ-synuclein toxicity in cells.
The observed phenotypes in the ɑ-synuclein-perfused chips reflect in vivo pathological conditions and add validity to the substantia nigra Brain-Chip as a suitable in vitro model of Parkinson’s disease.
Substantia nigra brain-chips reveal effects of ɑ-synuclein bundles on the blood-brain barrier
Several lines of evidence suggest that ɑ-synuclein bundles may originate elsewhere in the body before aggregating in the brain. To do so, however, these bundles would need to cross the blood-brain barrier. Notably, Parkinson’s disease patients are known to have a compromised blood-brain barrier, though the etiology of this symptom is not well understood. One possibility is that ɑ-synuclein bundles weaken endothelial tight junctions and contribute to barrier deterioration.
Using the substantia nigra Brain-Chip, the team of researchers sought to better understand the effect of ɑ-synuclein on the blood-brain barrier. To replicate distal production of ɑ-synuclein bundles and model delivery via systemic circulation, the vascular chamber—rather than the brain chamber—was perfused with the aberrant proteins for multiple days. The presence of these bundles in the vascular chamber resulted in substantial breakdown of the endothelial cell barrier. Staining for tight junction proteins decreased, and trans-chamber permeability increased markedly. Similar to cells in the brain chamber, endothelial cells presented with phosphorylated ɑ-synuclein and showed signs of inflammatory signaling, mitochondrial stress, and cell death.
Collectively, this suggests that ɑ-synuclein bundles contribute to the pathological breakdown of the blood-brain barrier and highlights the depth of data that can be gathered with this model. Whereas traditional two-dimensional cell cultures are limited in their ability to recreate complex cell-cell and cell-extracellular matrix interactions, the substantia nigra Brain-Chip makes it possible to gather a comprehensive and novel view of the neurovascular unit and its many cell types.
Attenuation of ɑ-synuclein bundle effects using trehalose
Given that ɑ-synuclein appears to induce blood-brain barrier defects and neuronal cell death through mitochondrial dysfunction, the team next assessed whether trehalose—an autophagy inducing therapeutic that has helped decrease protein aggregates and rescue mitochondrial function in other settings—could counter the effects of ɑ-synuclein.
Perfusion of the substantia nigra Brain-Chips with trehalose via direct tissue exposure or vascular exposure resulted in a substantial decrease in inflammatory signaling and cell death in the presence of ɑ-synuclein bundles as assessed by immunofluorescence and RNA-sequencing.
These experiments show how the Brain-Chip can be a valuable model for studying pharmacodynamics and pharmacokinetics within the neurovascular unit. By incorporating a brain-specific extracellular matrix component, dynamic fluid flow, and the co-culture of tissue relevant cell types, this model enables researchers to study the differential effects of therapeutics across the neurovascular unit, from endothelial cells to microglia.
Conclusions
In this comprehensive study, researchers describe a new model for Parkinson’s disease with significant potential for disease modeling and therapeutic development. By incorporating dopaminergic neurons, these researchers adapted the Brain-Chip to more closely resemble the in vivo conditions of the substantia nigra. Specifically, the substantia nigra Brain-Chip:
- Closely models the healthy substantia nigra, demonstrating sustained dopamine release from a phenotypically and transcriptomically mature cell population.
- Recreates cellular phenotypes that are characteristic of Parkinson’s disease, including inflammatory signaling, mitochondrial stress, breakdown of endothelial tight junctions, and neuronal cell death.
- Provided a detailed view of how trehalose—a lysosome inhibitor with potential application in neurodegenerative conditions—affects different cells in the neurovascular unit and demonstrated the potential of this model to aid in therapeutic drug development for Parkinson’s disease.
Now, more than 200 years after Dr. James Parkinson first pondered the cause of his patient’s tremors, the substantia nigra Brain-Chip offers researchers a viable and promising alternative to current Parkinson’s disease models that can be used to generate valuable insights into the molecular pathology of neurodegenerative disease.