Learn how these two complementary technologies can be combined for improved physiological relevance
In recent years, organoids—tiny, self-organized, three-dimensional cell models—have emerged as a promising technology for researching human physiology and disease. A major advantage of organoids is that they can be developed from induced pluripotent stem cells (iPSCs) or stem cells from primary human biopsies. As a result, they are able to differentiate into a variety of cell types to contain a greater range of cellular diversity than conventional models such as immortalized Caco-2 cell lines.
However, organoids lack some critical elements of the in vivo intestinal microenvironment, which limits their physiological relevance, such as the presence of vasculature and the mechanical forces caused by fluid flow and peristalsis. Additionally, their spherical structure results in several experimental challenges, including inconsistencies in size and shape, poor experimental control of key variables, and access to only one side of the epithelium.
Fortunately, researchers can unlock the full potential of organoids by using them as a robust cell source for Organ-Chips, enabling the creation of more accurate human biological models such as the Colon Intestine-Chip and Duodenum Intestine-Chip. Combining these technologies improves the organoids’ cellular morphology and functionality, results in more in vivo-like gene expression, and opens the door for new experimental designs—from simple drug permeability assays to more complex studies of colon inflammation, immune cell recruitment, and colorectal cancer tumor cell invasion.
Stay Up to Date with the Emulate newsletter
Combining Organ-Chips and Organoids
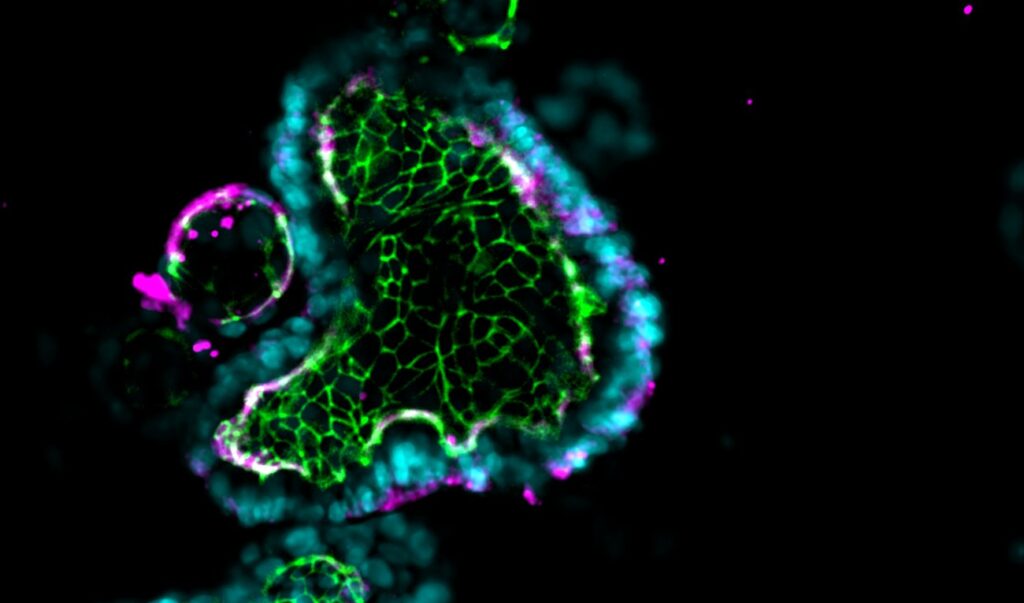
Each Emulate Organ-Chip contains a top (epithelial) channel and a bottom (endothelial) channel separated by a thin, flexible, porous membrane that enables cell-cell interaction. This membrane is coated with tissue-specific extracellular matrix (ECM) on top of which human cells can be cultured. To create the Intestine-Chip, intestinal epithelial organoids are first established from endoscopic biopsies of healthy adults.
They are then dissociated into fragments and seeded onto the ECM-coated porous membrane in the top channel. Meanwhile, primary microvascular endothelial cells are seeded on the other side of the ECM-coated porous membrane in the endothelial channel. Importantly, both the organoids and microvascular endothelial cells are intestine-specific, meaning researchers can model particular sections of the intestine, such as the duodenum, jejunum, ileum, or colon.
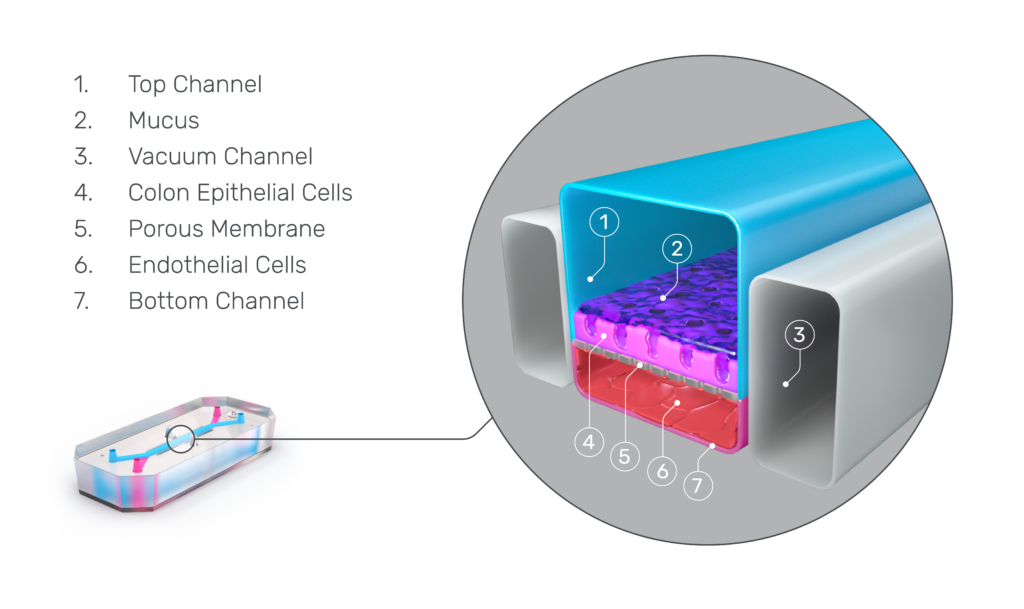
Distinct media flows through each channel to promote cellular differentiation, and stretch can be applied at different amplitudes and frequencies to create intestinal peristalsis-like motions. Over several days, the epithelial cells form a confluent monolayer in the top channel, and the endothelial cells form a complete blood vessel in the bottom channel.
The advantages of Organ-on-a-Chip technology
Using organoids as a cell source for Organ-Chips enables researchers to create more physiologically relevant models and allows for a greater range of study possibilities. Some of the advantages include:
Endothelial co-culture and tissue-tissue interactions

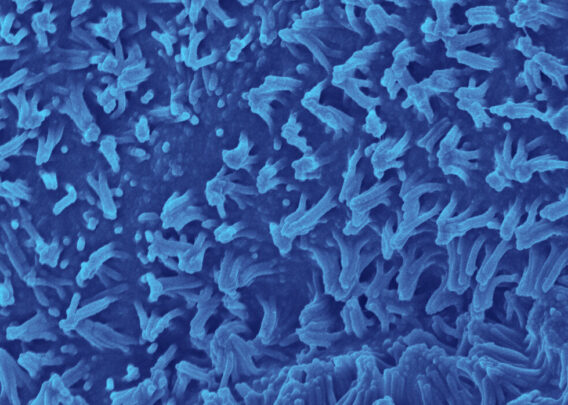
Organ-Chips allow for the inclusion of tissue-specific endothelial cells to recreate the epithelial-endothelial interface of the intestinal barrier and support tissue-tissue interactions—critical drivers of cellular function that organoids lack. This endothelial co-culture was shown to result in several distinct advantages in a study using the Colon Intestine-Chip, including enhanced epithelial polarity, correct localization of tight junction markers, a tight epithelial barrier with low permeability, and the formation of a mature brush border with densely packed and elongated microvilli. In addition, researchers can administer their drug candidate of interest through the vascular channel of Organ-Chips, recapitulating how many therapeutics reach the intended tissue in vivo.
Cell interactions and cytoarchitecture
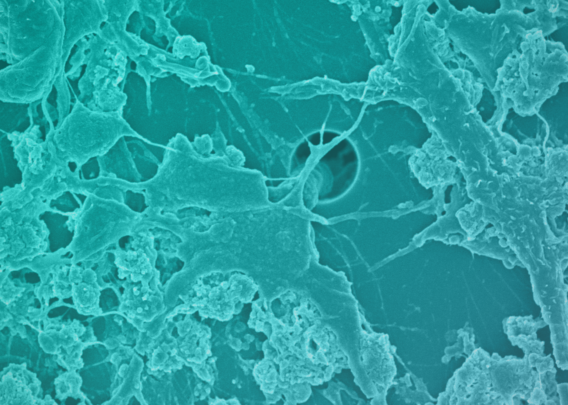
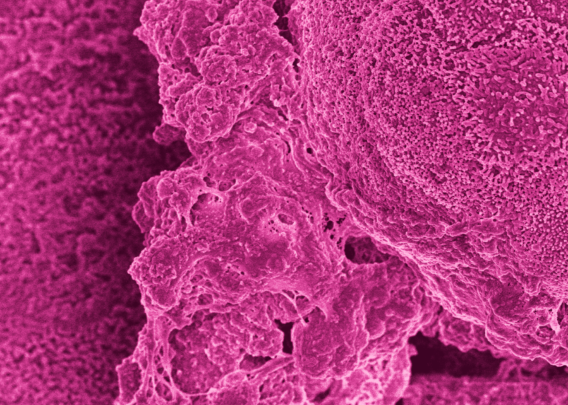
Although the cells in organoids are in a 3D structure, their organization does not resemble what it would be in vivo. The lack of directional cues results in somewhat random tissue organization, and the spherical shape results in reduced oxygen exposure in their center, often resulting in necrotic cores. In contrast, Organ-Chips provide the appropriate microenvironmental conditions for epithelial cells to spontaneously organize into physiological cytoarchitecture, including correct polarity and the formation of microvilli.
Dynamic media flow
Unlike organoids in static culture, Emulate Organ-Chips are designed to allow for continuous, unidirectional media flow, enabling steady-state nutrient levels and recreating the dynamic shear forces cells experience in the body. In the Duodenum Intestine-Chip, this media flow was shown to positively affect tissue architecture, resulting in increased cell height, cobblestone-like morphology, well-defined cell-cell junction formation, and dense microvilli.
Peristaltic-like stretching
With the Zoë Culture Module, researchers can fine-tune the frequency and strain of the chip’s flexible membrane to create peristalsis-like mechanical forces, enabling studies not possible with animals or alternative in vitro models. Recently, researchers at the Ellison Institute of USC applied this unique functionality to study the role of peristalsis in colorectal cancer tumor cell invasion. The Pasteur Institute has also leveraged this capability to study the impact of mechanical forces on Shigella infection and found that peristalsis is critical for specific stages of the infection process.
Improved gene expression
Multiple RNA-Seq analyses have shown that organoid-derived epithelial cells cultured in Emulate Organ-Chips have a transcriptome profile significantly closer to in vivo tissue than those same organoids in suspension. Analysis of specific pathways revealed differences in epithelial differentiation and key metabolic enzymes and pathways, indicating enhanced cell differentiation. This reinforces the advanced functionality of endothelial co-culture and the dynamic chip microenvironment. Given the closer in vivo gene expression, Organ-Chip models with organoids are more likely to express drug targets than organoids alone.
Immune cell incorporation
The fluidic nature of Emulate Organ-on-a-Chip technology allows users to introduce circulating immune cells through the chip channels, which is critical for modeling some aspects of disease. Additionally, research published in eLife used this approach to evaluate the safety of T-cell bispecific antibodies, a cancer immunotherapy difficult to study in animals due to fundamental species differences in immunological response.
Increase the physiological complexity of your organoid studies
Creating the next generation of effective therapeutics requires more human-relevant models of health and disease. While organoids offer several advantages over traditional monolayers, it is only when they are combined with Organ-on-a-Chip technology that their full potential can be realized, with improvements to cell morphology, functionality, and gene expression. By leveraging these advanced in vitro models, researchers can model more complex human biological mechanisms—including peristalsis, tumor cell migration, and immune cell interaction—enabling studies not possible with conventional models.
Contact us to learn more about how Organ-on-a-Chip technology can help you improve the physiological relevance of your research.
Not ready to chat? Check out our resources below to see data generated on the organoid-based Duodenum Intestine-Chip and Colon Intestine-Chip.