A summary of the paper published in iScience: A Microengineered Brain-Chip to Model Neuroinflammation in Humans
A growing and persistent storm of inflammatory signaling often accompanies diseases like Alzheimer’s, Parkinson’s, and Huntington’s—neurodegenerative diseases that affect more than a billion people worldwide. Decades of research have shined a light on the complex interplay among cell types of the brain that leads to the propagation of neuroinflammation. Yet, in spite of substantial advances in our understanding of the biology underlying neurodegenerative diseases, development of therapeutics that treat these conditions has not kept pace.
One potential reason for this discordance is the lack of human-relevant models on which to test new therapeutics. Because of their artificiality, conventional, two-dimensional cell cultures fail to capture the complexity of the brain neurovascular unit and the many cell-cell interactions that mediate a drug’s effects. Additionally, myriad differences between species in neurobiology limit the physiological relevance of animal models.
In a recent study published in iScience, Emulate researchers describe a new model of neuroinflammation based on Organ-on-a-Chip technology. By co-culturing multiple human brain cell types in a three-dimensional microphysiological system, the authors were able to more accurately recreate the structure and function of the human neurovascular unit and present a physiological model of neuroinflammation with significant advantages over contemporary models. Such a model may prove to be a critical tool in the development of novel therapeutics for neurodegenerative conditions.
Experimental Overview
Research Area: Brain; mechanisms of neuroinflammation in neurodegenerative diseases
Organisms: Human
Sample Types: Brain-Chip
Research Question: Can a human Brain-Chip accurately model human brain neuroinflammation?
Results:
- Microengineered human Brain-Chips develop mature excitatory and inhibitory cortical neurons juxtaposed with glia cells, astrocytes, pericytes, and a tight layer of endothelial-like cells; collectively, these mimic protein expression, permeability, and architecture of the in vivo human neurovascular unit (NVU).
- Relative to conventional Transwell cell culture systems, the Brain-Chip shows an RNA expression profile that more closely resembles adult human cortex tissue (a first for microphysiological systems).
- Perfusion of the Brain-Chip with TNF-α elicits key inflammatory features seen in neurodegenerative disease patients, such as glial activation, the release of proinflammatory cytokines, and increased barrier permeability.
Conclusion: Collectively, results from this paper indicate that the Emulate Brain-Chip more closely mimics human cortical tissue relative to alternative models. As such, this “brain-on-a-chip” model may help researchers better understand the biology of human cortical tissue and be used as a tool to help elucidate how either pathogenic signaling or therapeutics influence cortical tissue health.
Emulating the Neurovascular Unit
Neurodegeneration is driven, at least in part, by the establishment of a proinflammatory microenvironment. Compelling evidence suggests that local stress or systemic inflammation can compromise the blood brain barrier, enabling the entry of pro-inflammatory cytokines into the neurovascular unit and subsequent neuroinflammation. Additionally, reactive astrocytes and activated microglia are strongly implicated in blood-brain barrier breakdown and in the establishment of a neurodegenerative microenvironment.
The complex interactions of each cell type within the neurovascular unit appear to be critically important in driving disease progression and are also exceedingly difficult to model. Contemporary in vitro models range from two-dimensional co-cultures to three-dimensional multicellular organoids. However, no system has been able to simultaneously replicate neurovascular structure, function, and cell heterogeneity—all important factors that may affect cell behavior and therapeutic effectiveness.
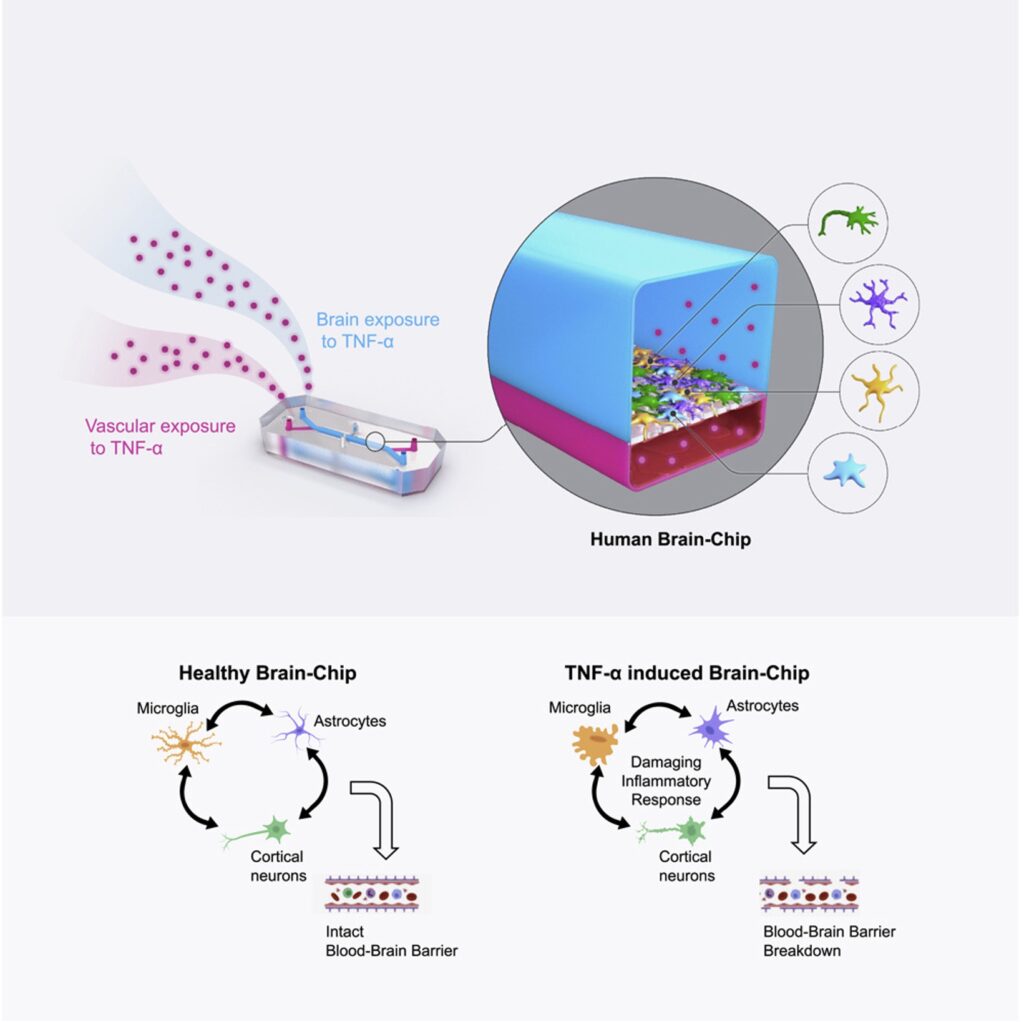
To address this challenge, Emulate researchers are developing the Brain-Chip, which contains human primary astrocytes, primary pericytes, iPSC-derived brain microvascular endothelial-like cells, iPSC-derived cortical neurons, and a human microglial cell line. This Brain-Chip was designed to have two chambers representing vascular tissue (consisting of iPSC-derived brain microvascular endothelial-like cells) and the brain parenchyma (with excitatory and inhibitory cortical neurons, microglia, astrocytes and pericytes).
Notably, this is the first such commercially available in vitro microphysiological system of the neurovascular unit to incorporate microglial cells. As mentioned above, microglia and astrocytes are believed to be significant drivers of neuroinflammation and are thus critical when modeling neurodegenerative diseases.
Before replicating pathological conditions, the team had to first show that the Brain-Chip accurately reflected the human cortical neurovascular unit. Immunofluorescent analyses of the chips revealed markers of excitatory and inhibitory cortical neurons alongside microglia, astrocytes, pericytes, and a monolayer of endothelial-like cells, indicating successful formation of the neurovascular unit. Functional maturation of the neurovascular unit was confirmed by measuring glutamate neurotransmitter levels and low barrier permeability, the results of which closely paralleled in vivo measurements.
Additionally, RNA sequencing analysis showed that gene expression profiles for cells cultured in the Brain-Chip were far closer to in vivo human cortical tissue cells than the same cells cultured in a Transwell model, demonstrating the superiority of the Brain-Chip as an advanced in vitro model system.
Collectively, these results indicate that the Emulate Brain-Chip is the first commercially available microphysiological system to comprehensively and physiologically model the human neurovascular unit.
Modeling Neuroinflammation
Having established the Brain-Chip as an accurate model of the human neurovascular unit, the next step was to assess its potential as a model for neuroinflammatory conditions.
The inflammatory cytokine TNF-α plays an important role in brain homeostasis, helping to regulate physiological processes such as learning and memory in healthy individuals. However, elevated levels of TNF-α can have pathological consequences. Alzheimer’s, Parkinson’s, and other neurodegenerative disorders have all been associated with high levels of TNF-α; however, it’s not yet clear how this cytokine elicits its many effects. One promising possibility is that TNF-α drives neuroinflammation through its effects on glial cells (astrocytes and microglia).
In the present study, researchers applied the Brain-Chip to study the mechanisms underlying TNF-α induced neuroinflammation within the neurovascular unit.
Perfusion of either the vascular-like channel or the brain parenchyma channel with TNF-α resulted in decreased levels of GLUT-1 (a well-known indicator of neurodegeneration), barrier disruption, astrocyte and microglia activation, and increased release of myriad proinflammatory cytokines—all characteristic indicators of neuroinflammation.
One of the benefits of this model is it allows for the capture of high-resolution data, enabling researchers to trace the contributions of individual cell types to the inflammatory niche. To this point, experiments using the Brain-Chip revealed that glial cells have a substantial impact on the release of proinflammatory cytokines such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and interferon-gamma (IFNγ). Removal of one or both cell types altered the tissue’s inflammatory phenotype in measurable ways, shedding new light on neuroinflammatory pathogenesis. Gathering such detailed observations was only possible because, unlike most in vitro systems, the Brain-Chip incorporates heterogeneous cell types, including glial cells.
Conclusion
Findings from this study determined that the Emulate Brain-Chip can successfully model human neuroinflammation, an important aspect of many neurodegenerative diseases. With that in mind, researchers can use the human Brain-Chip to:
- Advance understanding neuroinflammation’s mechanisms
- Identify new targets for neurodegenerative disease drug treatment
- More accurately evaluate the efficacy of anti-inflammatory drug candidates, helping to reduce the extremely high clinical trial failure rate seen for neurodegenerative disease
Ultimately, the Brain-Chip represents a more human-relevant model in which researchers can investigate the pathological processes associated with neurodegeneration. This means researchers can not only gain a better window into human health and disease, but also test therapeutics in an advanced, physiologically relevant environment. Such a model may help to de-risk drug discovery efforts and accelerate the development of effective and safe therapeutics.