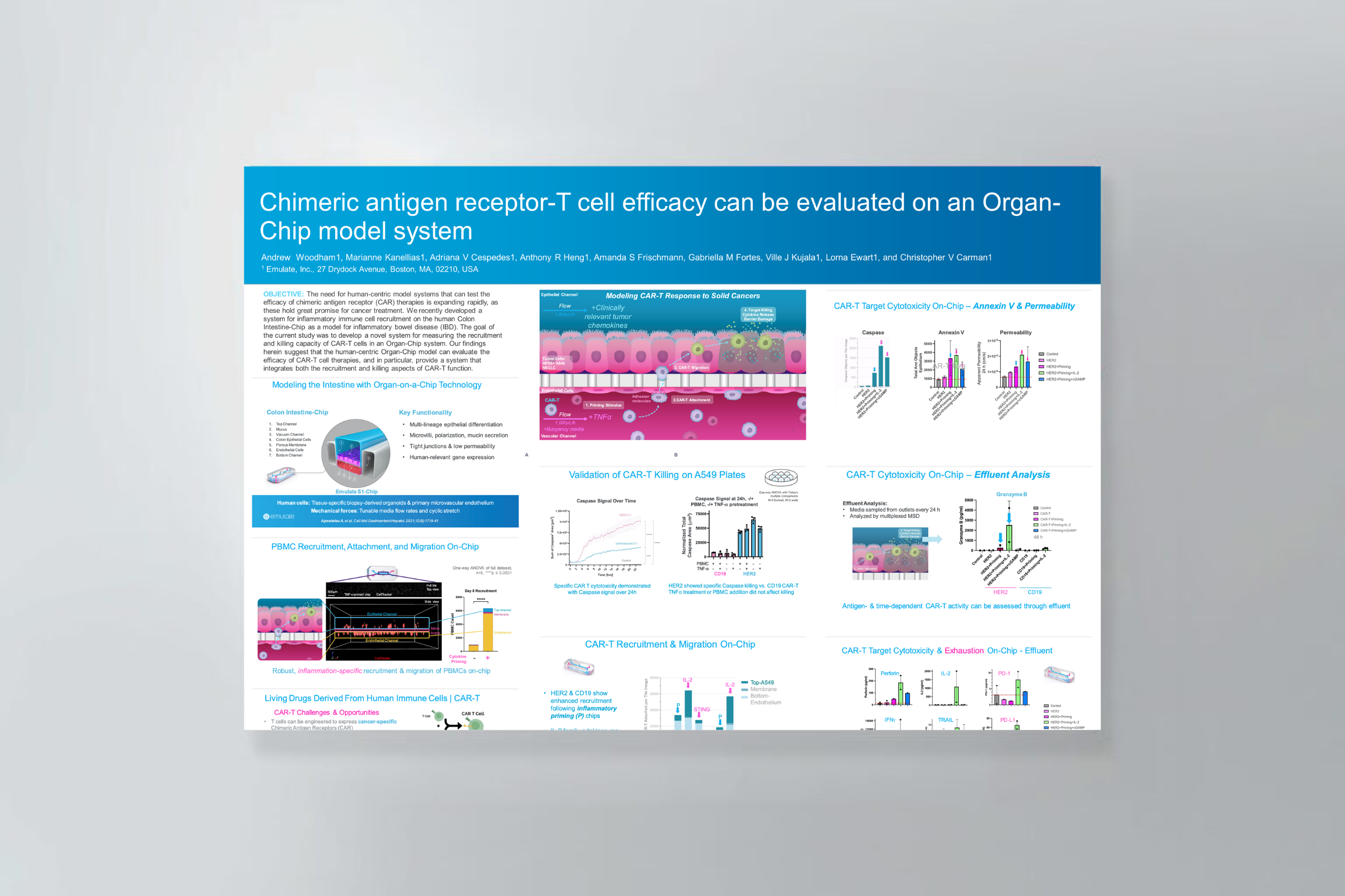
Chimeric antigen receptor (CAR) T-cell therapy shows significant potential in treating various human cancers. Unfortunately, researchers have faced difficulty in adapting this therapy to target solid tumors, where CAR T cells face unique challenges, such as antigen heterogeneity, an immuno-suppressive microenvironment, and T-cell exhaustion.
However, none of these challenges matter unless the CAR T cells can extravasate out of the blood stream and successfully migrate to the site of the tumor—an often-overlooked yet critical rate-limiting part of the process that cannot be modeled in conventional cell cultures.
This application note explains how Organ-Chips can be used to model the entire journey of solid tumor CAR T-cell therapy in a vascularized cancer cell line model.
In this application note, you will learn how Organ-Chips can be used to:
- Evaluate CAR T-cell vascular recruitment and killing efficacy in a single, unified assay.
- Assess antigen-specific killing and degranulation through a range of imaging and effluent-based analysis.
- Investigate co-therapeutic efficacy, as shown by proof-of-concept IL-2 data.
- Adapt the workflow to study a diverse range of solid tumor cell lines, immunotherapies, and co-therapeutics.