The Origins of Modern Cell Culture
The tone of Robert Hooke’s writing in 1665 was one of unmistakable glee. Hooke, having recently constructed a rudimentary but powerful microscope, was now able to document in detail the underlying components that afforded plants, rocks, and animals their functional properties. To Hooke, this newfound world held the key to understanding the nature of life.
“And could we so easily and certainly discover the [design] and texture even of these films and of several other bodies…we might as readily render the true reason of all their phenomena.”
Driven by this potential, Hooke proceeded to catalogue the microscopic world around him. His work, documented in Micrographia, not only introduced the term ‘cell’, but also demonstrated the benefits of meticulously studying the cellular world, thus laying the groundwork for modern cell biology.
Today, cell culture, which involves growing cells outside of their native organism, is a foundational tool in the molecular sciences. Cells that have been isolated from a patient or immortalized long ago allow researchers to study various aspects of the human body, using the cells as proxies for the larger organs they hail from. However, deriving valuable information about an organism from its cells is far from simple. Cells isolated from the human body (in vitro) are unlikely to behave naturally, and those within the body (in vivo) are often difficult to observe. There have been significant strides towards understanding the mechanics of life and disease at the cellular level, and recent advances in the technology used to study cells are opening up even newer possibilities.
In vitro cell culture involves growing living cells in a highly controlled, non-living environment. In vitro is Latin for “in the glass,” and as the name suggests, this kind of cell culture is most often carried out by growing cells in a two-dimensional (2D) plane on glass or plastic petri dishes. These cells may be sourced directly from patients (known as patient-derived cells) or collected from a patient long ago and subsequently engineered to enable long-term propagation (so called immortalized cell lines).
In Vitro Cell Culture Advantages and Disadvantages
Advantages
- Growing and maintaining cells is relatively inexpensive.
- Researchers gain a high degree of control over the cell’s environment, maintaining control over the nutrients, temperature, and other variables that cells are exposed to.
- These cells are often far easier to observe through a microscope, enabling high-content studies of the cell’s behavior.
- This approach is often amenable to high-throughput applications, such as drug or functional screening.
Disadvantages
- The in vitro environment is far removed from the cell’s natural environment in the human body, where cells experience three-dimensional contact with proteins and other cells, biomechanical forces, as well as dynamic nutrient and waste gradients. Each of these factors can influence how the cell behaves. By removing cells from a complex environment to a far more simplified one, the translational value of in vitro cell culture diminishes.
- In vitro cell culture may result in artificial mutations that cause cells to behave abnormally.
In Vivo Studies: Advantages and Disadvantages
In vivo, meaning “within the living,” involves studying cells within their native organism. This method provides the most accurate representation of how cells behave in their physiological context. In vivo studies have long been viewed as the gold standard for understanding complex interactions within tissues, organs, and systems, as well as for assessing the real-world effects of drugs and treatments. This is largely because of the aforementioned drawbacks of traditional in vitro cell culture systems.
However, in vivo studies come with a significant cost. The in vivo environment is inherently more complex, making it difficult to isolate the effects of specific variables on cell and organismal behavior. Additionally, many in vivo studies are performed in model organisms, such as rats and dogs, to predict how the human body will respond to disease or therapeutics. The genetic and physiological differences between animals and humans can erode the accuracy of these models. This is particularly hazardous in preclinical drug safety testing, where inaccurate predictions can lead to dangerous compounds advancing into the clinic.
Bridging the gap with more advanced in vitro systems
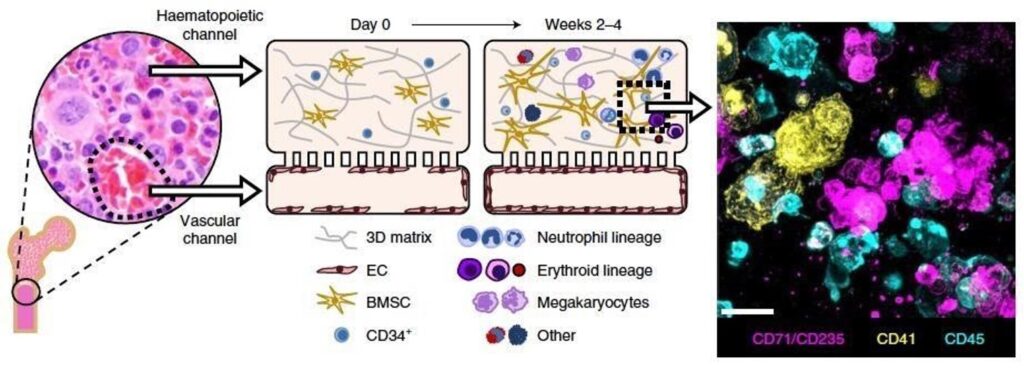
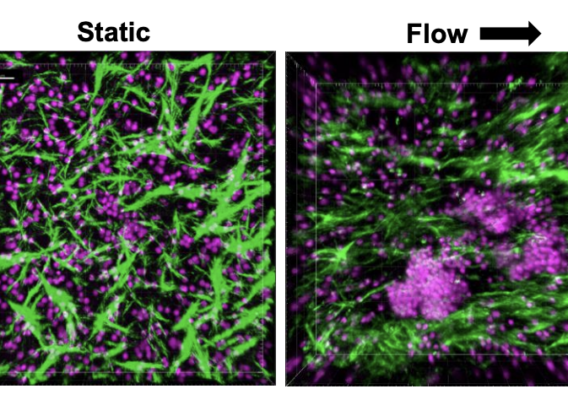
Recent advances to in vitro cell culture are helping to alleviate the challenges researchers have faced historically, particularly by incorporating more complex, in vivo-relevant environments. The most powerful example of this comes from Organ-on-a-Chip technology, also known as “Organ-Chips.”
Organ-Chips are advanced, three-dimensional in vitro culture systems that closely mimic the natural environment of a cell. Specifically, these systems expose cells to biomechanical forces, dynamic fluid flow, and heterogenous cell populations while providing three-dimensional contact with proteins or other cells. Collectively, these features encourage the cells to behave as they would in a living organism, thus greatly improving researchers’ ability to accurately study the behavior of cells in vitro. And, unlike many in vivo model systems, Organ-Chips can be constructed with human cells, thus circumventing the interspecies differences that plague many in vivo systems. As such, Organ-Chips are now being used in a variety of applications to help researchers study cell behavior with increasing accuracy.
Cell biology has evolved considerably since Hooke’s discovery, progressing in ways that allow researchers to study the cellular world with ever greater accuracy. That progression has often been catalyzed by technological development, from Hooke’s microscope to the advent of Organ-on-a-Chip technology.
Commonly Asked Questions About Modern Cell Culture
What is Ex Vivo Cell Culture?
Ex vivo cell culture is a form of in vitro cell culture that uses cells or tissue freshly collected from a living organism. Ex vivo cell culture may be particularly valuable when studying patient-specific conditions or when working with mature, terminally differentiated cell types that are difficult to produce with stem cells.
Are in vitro studies reliable?
Yes, to an extent. In vitro studies can be extremely valuable tools for studying cell biology and human physiology. While the traditional in vitro cell culture environment is very different from an in vivo environment—and thus cells are less likely to behave as they would in vivo—many cellular phenotypes are quite robust. This means that some cell behaviors in vitro do accurately represent how cells in vivo would behave. However, the reliability of in vitro studies can be greatly improved by using more complex culture systems, such as Organ-Chips, that faithfully emulate the cells’ natural environment.
What is a typical in vitro study design?
In vitro studies can be vastly different in design and scale; therefore, there is no single template for an in vitro study. However, there are common elements within subdisciplines. For example, in vitro studies for pharmaceutical development often use human cell lines (for larger scale studies, immortalized cells are common early in the drug development process). These cells may be grown in 96-well plates that allow for automated handling. Candidate drugs may then be added, one to each well of cells, followed by a period of observation. The cell’s ability to survive, proliferate, or release various signaling factors may be measured (among many potential response mechanisms).