With over 130 peer-reviewed publications across 30+ organ models, Emulate Organ-Chips are empowering researchers to make game-changing scientific breakthroughs! Download this digest to easily explore all publications related to your field of research, or to just learn more about how the technology itself is developing.
New this quarter:
Bone (Metastasized Breast Cancer)
- Multi-omics qualification of an organ-on-a-chip model of osteolytic bone metastasis
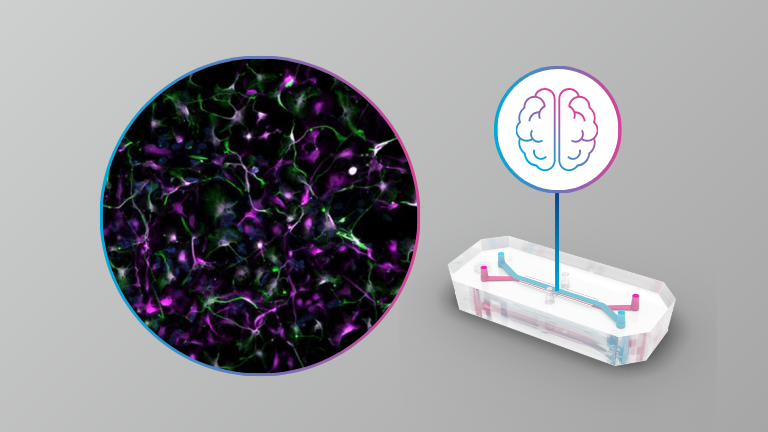
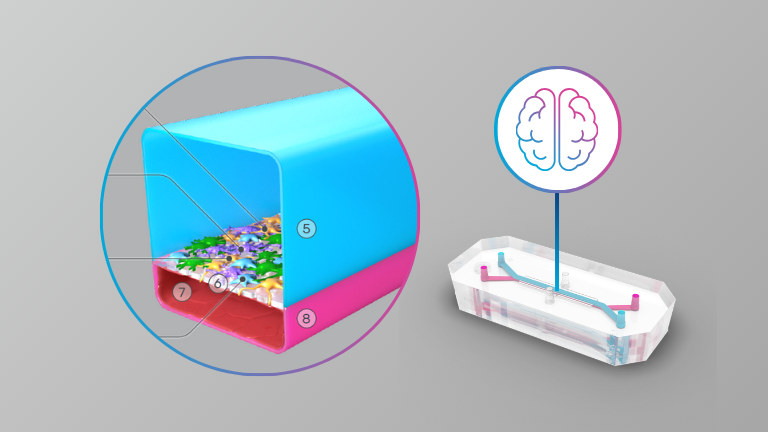
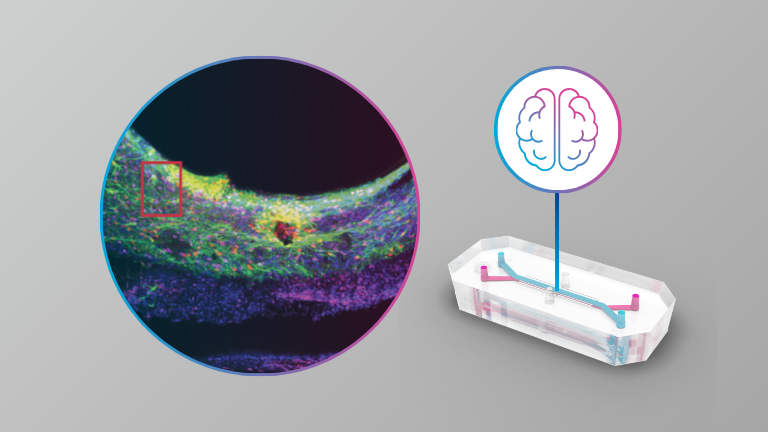
Brain
- A guided approach to establish a functional humanized Brain-on-a-Chip microfluidic model of the neurovascular system
Intestine
- Gene expression profiling reveals enhanced nutrient and drug metabolism and maturation of hiPSC-derived intestine-on-chip relative to organoids and Transwells
- Establishing the human Duodenum-Chip as a surrogate for effective human permeability: In vitro and in silico assessment
Liver
- Challenges and solutions in measuring commonly used biomarkers for drug-induced liver injury in a Liver-On-A-Chip platform
Lung
- Lung microphysiological system validates novel cell therapy for Acute Respiratory Distress Syndrome
- Revealing the impact of Pseudomonas aeruginosa quorum sensing molecule 2’-aminoacetophenone on the human bronchial-airway epithelium and pulmonary endothelium using a human airway-on-a-chip
Vasculature
- Human coronary artery tri-culture organ-chip recapitulates anti-inflammatory effect of pulsatile wall strain