Organ Model: Lung (airway)
Application: Immunology & Inflammation, Cancer
Highlights
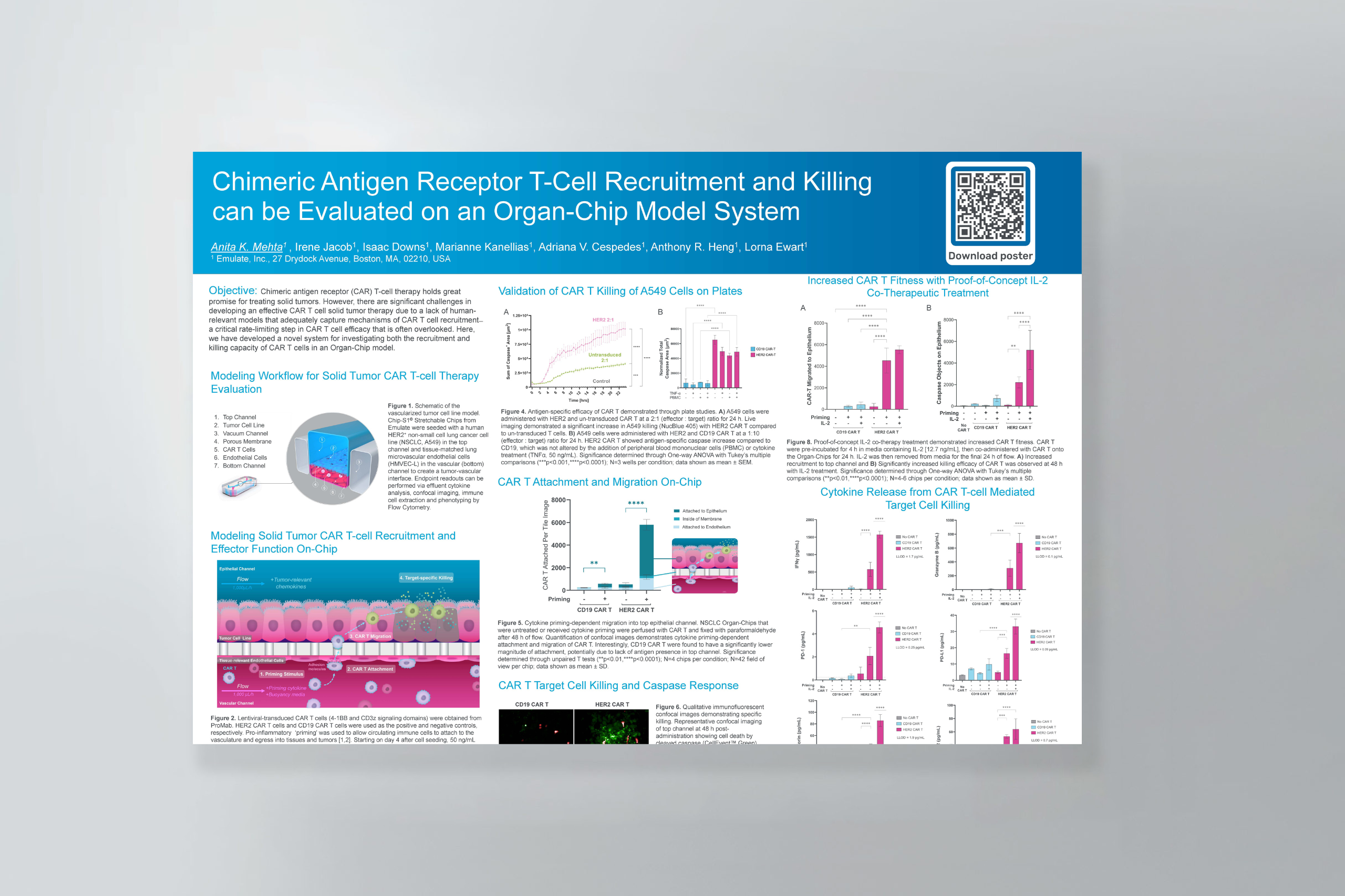
The authors of this study leveraged Organ-Chips to model cystic fibrosis airways. The researchers used CRISPR/Cas9 to knock out CFTR in human bronchial epithelial cells, then grew them in Chip-S1 Organ-Chips to study how CFTR loss drives inflammation, immune cell recruitment, and epithelial invasiveness — key features of CF lung disease. Compared to wild-type controls, CFTR-deficient chips showed increased recruitment of polymorphonuclear neutrophils and greater migration of epithelial cells into the endothelial channel, reflecting a pro-inflammatory, EMT-associated phenotype. These chip-based functional assays complemented proteomic findings, linking CFTR loss to pathways involved in cancer progression and immune cell recruitment.