By Jim Corbett, CEO, Emulate

Drug discovery is at a crossroads. With nearly 90% of clinical trial candidates failing to reach FDA approval, the need for more predictive, human-relevant models has never been greater. That’s why Emulate recently launched the AVA™ Emulation System, the first self-contained Organ-on-a-Chip workstation designed to bring scale, reproducibility, and accessibility to this transformative technology. As the company’s CEO, Jim Corbett brings a unique perspective on how AVA will accelerate adoption across pharma, biotech, and academia—and why it represents a turning point for human-relevant drug discovery. In this blog post, Jim shares how AVA was built to overcome longstanding barriers in the field and what its introduction means for the future of drug development.
Rethinking Preclinical Models
The pharmaceutical industry faces a staggering challenge: nearly 90% of candidate drugs that enter clinical trials fail to gain FDA approval. Traditional reliance on animal models has proven insufficient for predicting human responses, leaving researchers searching for more accurate and efficient solutions.
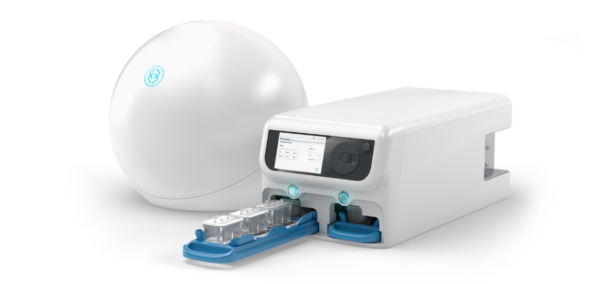
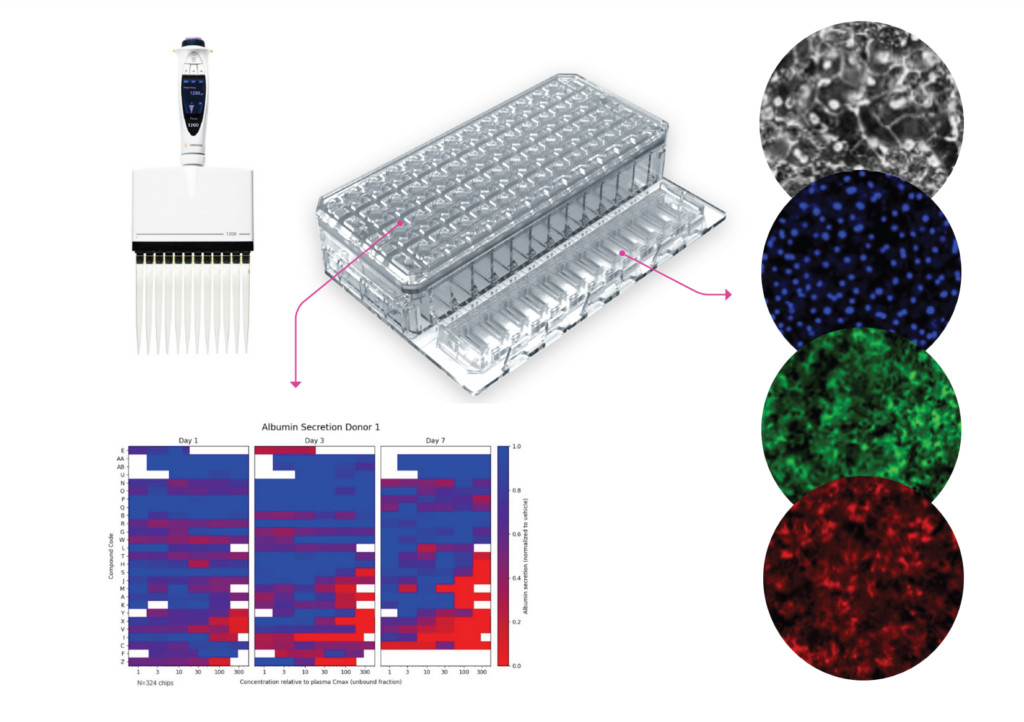
Organ-on-a-Chip technology offers a transformative alternative by modeling human biology with unprecedented fidelity. Yet for years, adoption was constrained by scale and cost. That’s where AVA comes in. Designed as the first self-contained Organ-on-a-Chip workstation, AVA integrates high-throughput microfluidic culture, full environmental control, and real-time imaging—all within a compact benchtop unit. Supporting up to 96 Organ-Chip Emulations in a single run, AVA delivers insights at a scale that makes Organ-Chips viable as a standard tool in preclinical workflows.


Building Confidence Through Scale and Reproducibility
For Organ-on-a-Chip technology to impact drug development meaningfully, the data must be both accurate and reproducible. AVA enables exactly that. By allowing large-scale experiments, it empowers scientists to generate robust datasets that validate predictive models for efficacy and safety. The result: researchers can make more confident decisions about which drug candidates to advance, reducing costly late-stage failures.

From Liver-Chip to AVA: A Foundation of Regulatory Trust
A landmark 2022 study in Communications Medicine demonstrated the predictive power of Emulate’s Liver-Chip in identifying drug-induced liver injury. That study not only paved the way for the Liver-Chip’s inclusion in FDA’s ISTAND program but also set the benchmark for AVA.
Building on that foundation, AVA incorporates equivalency studies designed to show performance on par with or better than previous generations of Organ-Chip technology. By combining this proven biological fidelity with higher throughput, AVA strengthens regulatory confidence while accelerating the path to qualification as a recognized tool for drug development.


Empowering Pharma to Capitalize on Regulatory Shifts
Regulators are now explicitly encouraging the inclusion of New Approach Methodologies (NAMs) like Organ-Chips in IND submissions. With AVA, pharmaceutical companies can generate human-relevant data at the throughput and scale required for regulatory acceptance.
Importantly, AVA also complements other NAMs—such as computational modeling and omics-based approaches—by providing organ-level validation. This synergy creates a weight-of-evidence approach that strengthens submissions and supports the global movement toward reducing animal testing.


Making Human-Relevant Research Accessible to Academia
While industry adoption is critical, academic research plays a pivotal role in innovation. Historically, cost has limited Organ-Chip use in academic settings. AVA changes this dynamic by reducing the cost per sample by more than 75%, making it feasible for academic labs and core facilities to run Organ-Chip experiments regularly. The result is broader access to human-relevant models that can shape early-stage discoveries and future therapies.

Overcoming Persistent Barriers: Scale, Cost, and Workflow Efficiency
High-throughput and reproducibility have long been bottlenecks in Organ-on-a-Chip research. AVA was designed to overcome these barriers by automating workflows, integrating imaging directly into the system, and enabling seamless compatibility with robotic liquid handlers. This means researchers can scale experiments without sacrificing quality or disturbing biological conditions, producing harmonized datasets suitable for regulatory and industrial pipelines.

Shaping the Future of Drug Discovery
Looking ahead, AVA has the potential to transform how pharma and biotech approach drug discovery. From helping companies like Moderna pre-screen lipid nanoparticles for safety, to accelerating early-stage research in academic labs, AVA is enabling more efficient, cost-effective, and human-relevant workflows.
The convergence of regulatory change and technological innovation makes this moment especially pivotal. With the FDA and NIH shifting expectations toward human-relevant data, Organ-on-a-Chip technology—uniquely capable of recapitulating organ physiology—stands at the forefront of this new era. By delivering high-fidelity datasets that fuel both regulatory decision-making and AI-driven predictive models, AVA is not just advancing science—it’s redefining the standard for drug discovery.
Ready to future-proof your pipeline?
Explore our portfolio of sophisticated and user-friendly platforms that make it easy to get started with Organ-on-a-Chip technology.