Originally presented at the MPS World Summit 2025 Annual Meeting in Brussels, Belgium.
Authors
Naomi S. Coombes1, Tanja Šuligoj2, Rhiannon Davies1, Tessa Prince2, Lisa Luu2, Conner Norris1, Julian Hiscox2, Yper Hall1, Simon GP Funnell3, Kevin R. Bewley1
1UK Health Security Agency, Porton Down, Salisbury, UK; 2Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, Liverpool, UK; 3Gut Microbes and Health, Quadram Institute Bioscience, Norwich, UK
Abstract
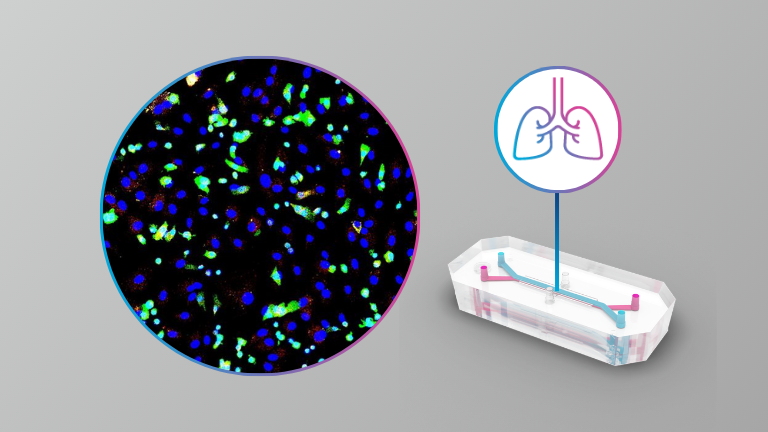
The SARS-CoV-2 pandemic caused significant worldwide disruption to economies and healthcare systems. It is predicted that the frequency at which new pandemics occur in the future will increase due to factors such as climate change, global population increase, intensification of agriculture and closer contact with wildlife. All but one of the pathogens on the current WHO R&D emergency context priority disease list are viruses which require work to be performed at containment level 3 or 4 (BSL3 or BSL4). There is a need for physiologically relevant in vitro systems to assist pre-clinical research in preparedness for the next pandemic. For example, Disease X which by definition will be a human disease, may not infect current cell lines or animal models commonly used in virus research.
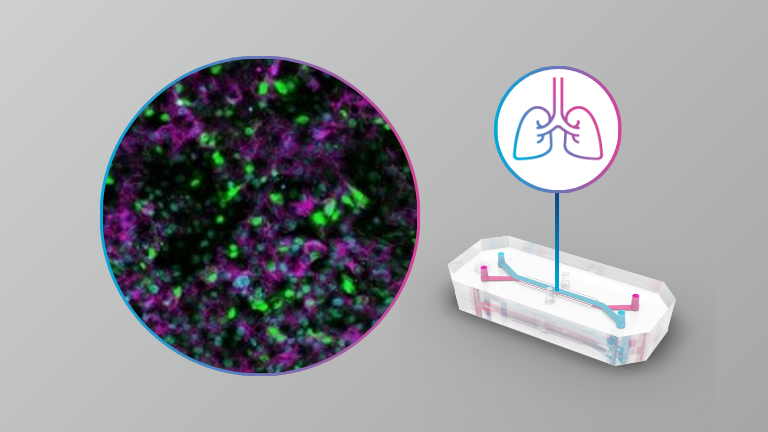
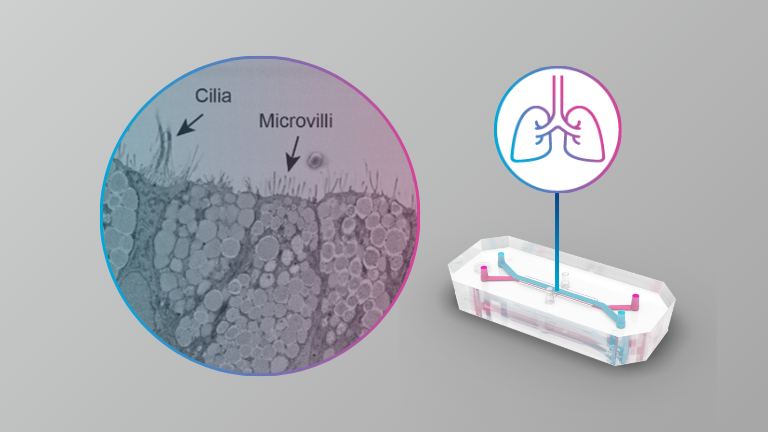
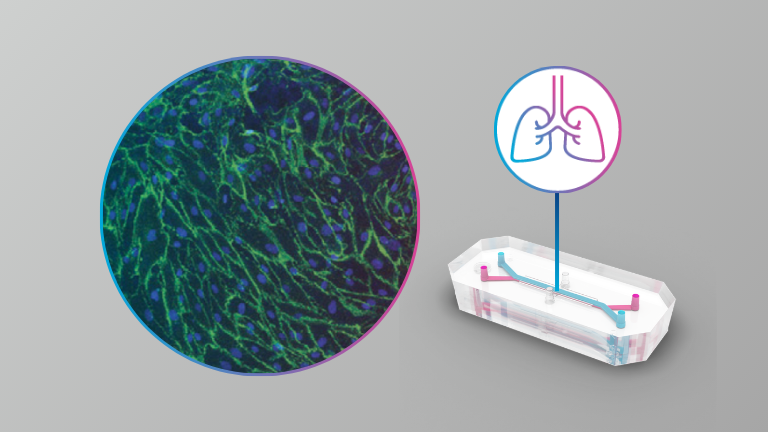
Here the authors present the results from infections with SARS-CoV-2, SARS-CoV-1 and MERS-CoV of an alveolar model using the commercially available Emulate system at BSL3. They also describe the results from antiviral assessment in this model.