Women’s reproductive health has historically been underserved in biomedical research. Conditions such as bacterial vaginosis, cervical infections, and heavy menstrual bleeding affect millions worldwide, yet remain poorly understood and under-treated. One major reason is that conventional preclinical models—animal studies and static cell culture—cannot capture the complex, hormone-responsive biology of the human female reproductive tract. As Dr. Donald Ingber once famously quipped, “mice don’t menstruate.” Thus, without a good model, advancements in the understanding and treatment of women’s reproductive health have continued to lag.
Organ-Chip models, also called microphysiological systems (MPS), are helping to close this critical gap. By recreating human tissue microenvironments in a dynamic, microengineered platform, Organ-Chips enable scientists to investigate reproductive health with unprecedented physiological relevance. Below, we highlight three case studies—the Vagina-Chip, Cervix-Chip, and a new project focused on Uterus-Chips for heavy menstrual bleeding—that demonstrate how this technology is transforming women’s health research.
Modeling the Vaginal Microbiome: The Vagina-on-a-Chip
The vaginal microbiome plays a vital role in reproductive health, influencing susceptibility to infections, pregnancy outcomes, and sexually transmitted diseases. Yet, until recently, researchers lacked a human-relevant model to study how microbial communities interact with vaginal tissue.
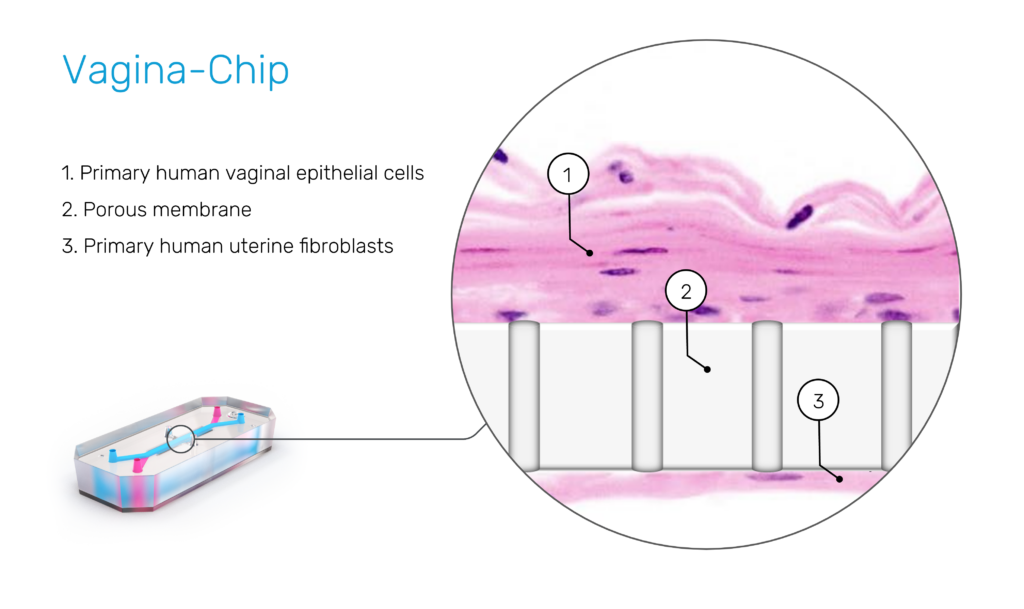
In 2022, researchers published the first Vagina-on-a-Chip in Microbiome. This Organ-Chip was lined with primary human vaginal epithelial cells and perfused with culture media under controlled flow conditions, mimicking the dynamic vaginal environment. The platform allowed direct co-culture of live microbial consortia with human tissue—something impossible with animal models.
Key findings included:
- Stable colonization by Lactobacillus crispatus, a beneficial species linked to vaginal health, which maintained a protective acidic environment.
- Dysbiosis-induced inflammation, where introducing a pathogenic community triggered epithelial damage and immune activation, closely mirroring bacterial vaginosis in patients.
As lead author Gautham Mahajan, PhD, explained:
“Our Vagina-Chip model provides an unprecedented opportunity to study host–microbiome interactions in a controlled human-relevant context. This platform can help us untangle how microbial imbalances drive disease and guide the development of targeted probiotics or other therapies.”
By directly modeling the vaginal microbiome, the Vagina-Chip creates a new preclinical platform to evaluate probiotic interventions, antimicrobial therapies, and microbiome-targeted strategies that could improve outcomes in women’s reproductive health.
Understanding Cervical Health: The Cervix-Chip
The cervix regulates fertility, pregnancy maintenance, and protection against infection, but has historically been neglected in preclinical research. Two recent Cervix-Chip studies demonstrate how Organ-Chip models can reveal hormone-dependent and immune-regulated mechanisms of cervical function.
Hormone Sensitivity and Immune Function
A 2024 Nature Communications publication used Cervix-Chips lined with epithelial and stromal cells to study the effects of cyclical hormones on cervical physiology. The chips replicated hormone-driven changes in:
- Mucus secretion, essential for regulating fertility and pathogen defense.
- Immune signaling, showing how estrogen and progesterone modulate innate immune pathways.
These insights have direct implications for understanding cervical infections, infertility, and preterm birth.
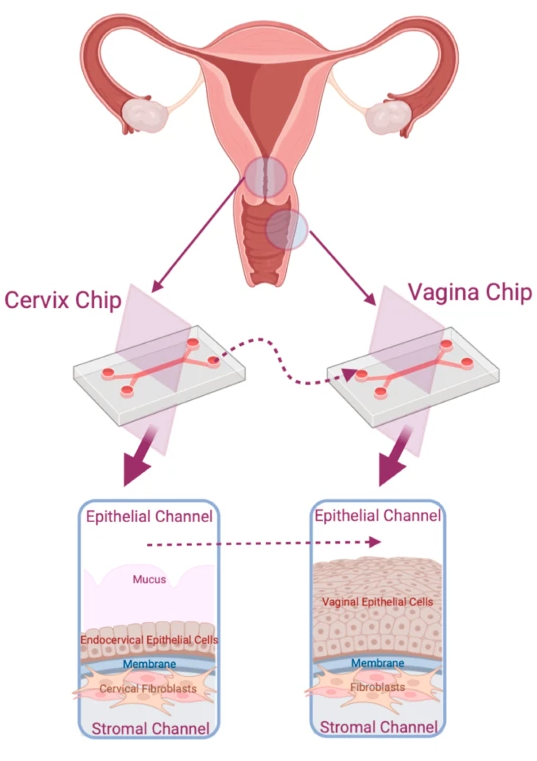
Crosstalk Between Cervix and Vagina
In 2025, researchers extended this work in npj Women’s Health by linking Cervix-Chips with Vagina-Chips. This integrated Organ-Chip system demonstrated how cervical mucus influences vaginal microbial balance. Disruption of mucus secretion allowed pathogenic bacteria to dominate, reproducing clinical patterns of dysbiosis and infection risk.
Together, these findings highlight how human-relevant preclinical models can uncover organ–organ interactions critical to women’s reproductive health—insights that were inaccessible with traditional animal models.
Tackling Heavy Menstrual Bleeding: A Uterus-on-a-Chip Approach
Heavy menstrual bleeding (HMB) affects nearly one in three women of reproductive age and is more prevalent than asthma or diabetes. It contributes to anemia, fatigue, and lost productivity, yet remains poorly understood because no animal models accurately reproduce menstruation.
Just this month, the Wyss Institute announced a Wellcome Leap–funded project to develop the first Uterus-on-a-Chip model of heavy menstrual bleeding. This project will build a microengineered system incorporating the endometrial lining, stromal cells, and vascular networks to replicate both normal menstruation and pathological bleeding.
The initiative aims to integrate:
- Multi-omics analyses, capturing transcriptomic, proteomic, and metabolomic signatures of abnormal bleeding.
- AI-driven data pipelines, accelerating biomarker discovery and therapeutic target identification.
Wyss Founding Director Dr. Donald Ingber emphasized the project’s potential:
“By creating the first physiologically relevant human model of heavy menstrual bleeding, we hope to not only uncover its root causes but also unlock new, life-changing treatments that can be delivered to patients in months rather than years.”
The ultimate goal is to reduce the average time to effective treatment for heavy menstrual bleeding patients from five years to just five months—accelerating progress in an area of women’s health that has been neglected for decades.
Why Organ-Chip Models Are a Turning Point for Women’s Health
The Vagina, Cervix, and Uterus Chips illustrate the broader promise of Organ-Chip technology in women’s reproductive health research:
- Human relevance: Organ-Chips use primary human cells under physiologically realistic flow and hormone conditions, avoiding species differences that limit animal studies.
- Dynamic microenvironments: Media perfusion and biomechanical cues replicate the living tissue environment, capturing complexity that static 2D cultures miss.
- System-level biology: Linking multiple Organ-Chips (e.g., cervix and vagina) enables the study of organ–organ interactions that shape reproductive health and disease.
- Therapeutic discovery: Integration with omics technologies and computational models accelerates identification of novel biomarkers, drug targets, and treatment strategies.
By addressing previously unanswerable questions—how microbial dysbiosis drives bacterial vaginosis, how cervical mucus supports fertility and infection defense, or how abnormal menstruation occurs—Organ-Chip models are redefining the landscape of women’s health research.
Looking Ahead
Organ-on-a-Chip technology is advancing from proof-of-concept models to regulatory-relevant preclinical platforms. For women’s reproductive health, this progress could not be more timely.
From microbiome research to cervical biology to menstrual disorders, Organ-Chips are generating actionable insights that could lead to:
- New non-hormonal treatments for heavy menstrual bleeding.
- Microbiome-based interventions to restore vaginal health.
- Improved diagnostics and preventive therapies for cervical infections and preterm birth.
These advances not only improve care for women but also strengthen overall public health. Better reproductive health research means healthier pregnancies, healthier families, and a more equitable healthcare system.
For too long, women’s health conditions were marginalized as too complex or too difficult to model. With Organ-on-a-Chip technology, those barriers are falling—chip by chip, breakthrough by breakthrough.
Ready to LEARN MORE?
Continue your learning by watching the on-demand webinar, “Assessing Reproductive Health with a Human Vagina-on-a-Chip”