Originally presented at the Society of Toxicology 2025 Annual Meeting.
Authors
Glen M. DeLoid, Davood Kharaghani, Zhenning Yang, Lila Bazina, Ping He, Ben Swenor, Trung Huu Bui, Nubia Zuverza-Mena, Carlos Tamez, Craig Musante, Michael Verzi, Jason C. White, and Philip Demokritou
Abstract
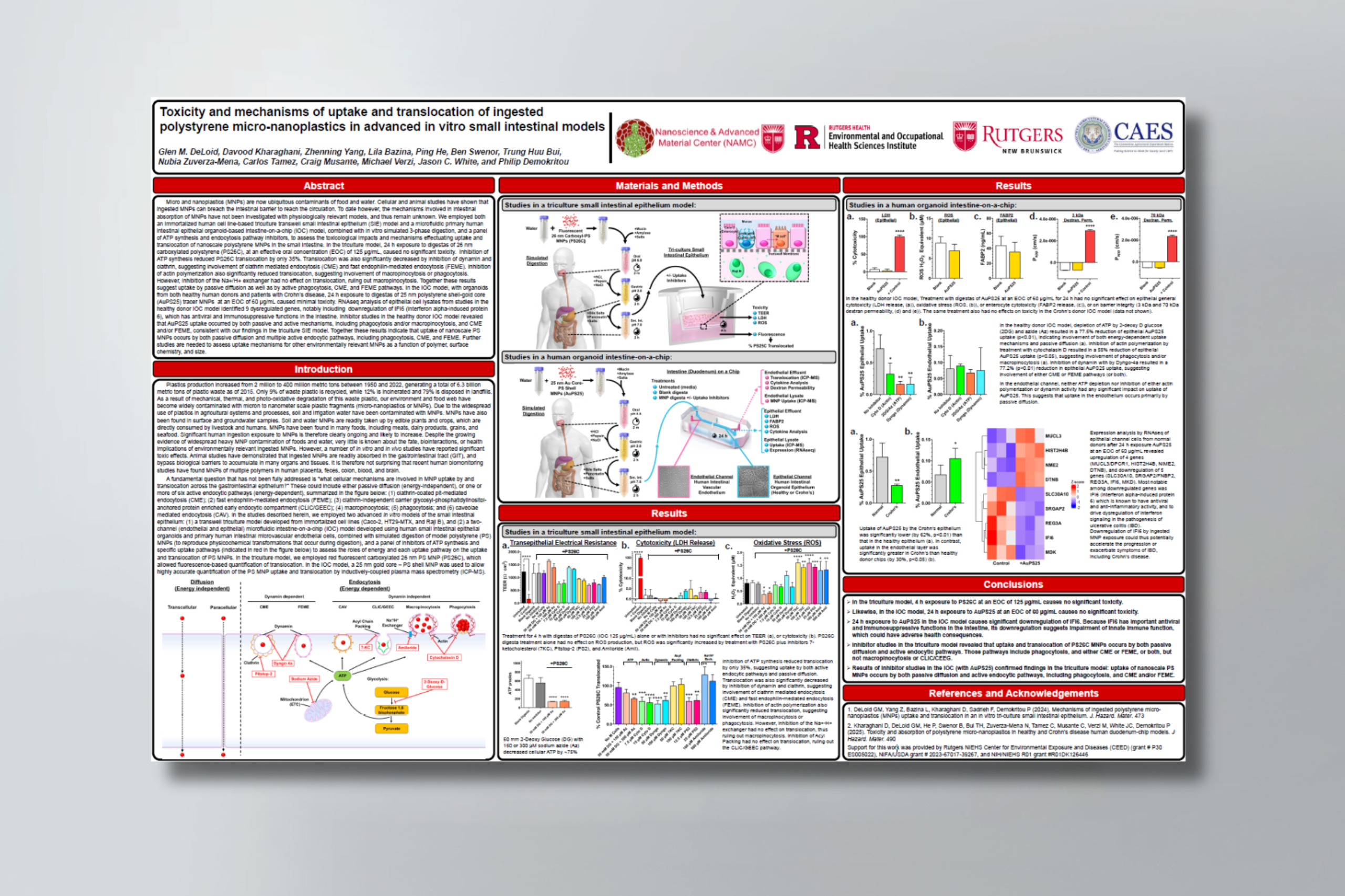
Micro and nanoplastics (MNPs) are now ubiquitous contaminants of food and water. Cellular and animal studies have shown that ingested MNPs can breach the intestinal barrier to reach the circulation. To date however, the mechanisms involved in intestinal absorption of MNPs have not been investigated with physiologically relevant models, and thus remain unknown. We employed both an immortalized human cell line-based triculture transwell small intestinal epithelium (SIE) model and a microfluidic primary human intestinal epithelial organoid-based intestine-on-a-chip (IOC) model, combined with in vitro simulated 3-phase digestion, and a panel of ATP synthesis and endocytosis pathway inhibitors, to assess the toxicological impacts and mechanisms effectuating uptake and translocation of nanoscale polystyrene MNPs in the small intestine. In the triculture model, 24 h exposure to digestas of 26 nm carboxylated polystyrene (PS26C), at an effective oral concentration (EOC) of 125 μg/mL, caused no significant toxicity. Inhibition of ATP synthesis reduced PS26C translocation by only 35%. Translocation was also significantly decreased by inhibition of dynamin and clathrin, suggesting involvement of clathrin mediated endocytosis (CME) and fast endophilin-mediated endocytosis (FEME). Inhibition of actin polymerization also significantly reduced translocation, suggesting involvement of macropinocytosis or phagocytosis. However, inhibition of the Na+/H+ exchanger had no effect on translocation, ruling out macropinocytosis. Together these results suggest uptake by passive diffusion as well as by active phagocytosis, CME, and FEME pathways. In the IOC model, with organoids from both healthy human donors and patients with Crohn’s disease, 24 h exposure to digestas of 25 nm polystyrene shell-gold core (AuPS25) tracer MNPs at an EOC of 60 μg/mL caused minimal toxicity. RNAseq analysis of epithelial cell lysates from studies in the healthy donor IOC model identified 9 dysregulated genes, notably including downregulation of IFI6 (interferon alpha-induced protein 6), which has antiviral and immunosuppressive functions in the intestine. Inhibitor studies in the healthy donor IOC model revealed that AuPS25 uptake occurred by both passive and active mechanisms, including phagocytosis and/or macropinocytosis, and CME and/or FEME, consistent with our findings in the triculture SIE model. Together these results indicate that uptake of nanoscale PS MNPs occurs by both passive diffusion and multiple active endocytic pathways, including phagocytosis, CME, and FEME. Further studies are needed to assess uptake mechanisms for other environmentally relevant MNPs as a function of polymer, surface chemistry, and size.
Related publication: Toxicity and absorption of polystyrene micro-nanoplastics in healthy and Crohn’s disease human duodenum-chip models