Introduction: What is PRECISION Medicine?
“Precision medicine” (also called personalized medicine) has become a guiding aspiration in healthcare: treating each person not as a generic patient but based on their unique biology, disease subtype, and treatment responsiveness. In its ideal form, clinicians would know in advance which therapy will work — and which ones won’t — for a given patient, thereby improving outcomes and reducing unnecessary treatments and potential toxicity.
Yet in practice, personalized medicine still faces major hurdles:
- Many therapies are selected on the basis of genomics or biomarkers only, but these approaches don’t always capture how the patient’s cells will actually behave when exposed to treatment.
- Current in vitro models used for personalized medicine (2D cell culture, simple organoids) often lack the complexity of the human tissue environment — including perfusion, stromal interactions, biomechanical forces, and multi-cellular cross-talk.
- Accessing meaningful patient-derived samples (especially for internal organs or complex microenvironments) is difficult, and replicating their physiology ex vivo is challenging.
This is where Organ-on-a-Chip technology is emerging as a powerful agent of change. By creating microfluidic devices lined with human cells and recreating organ-specific tissue architecture, fluid flow, and cell-cell interactions, Organ-Chips offer the most realistic “home away from home” for patient samples. These systems can more faithfully reproduce how a patient’s cells behave in situ, making them a promising bridge from patient-derived sample to clinical decision.
In this blog, we explore how Organ-Chips are being used in personalized medicine, and then dive into three compelling case studies that illustrate how they are making a real-world difference.
How Organ-on-a-Chip Technology Elevates Patient-Derived Models
Organ-on-a-Chip technology recreates the functional unit of an organ using living human cells and an organ-specific microenvironment, giving researchers a real-time view of human biology. These small devices consist of two parallel channels, which can be seeded with multiple human-relevant cell types—including primary cells, iPSCs, organoids, and immune cells—and are separated by a thin, porous membrane that allows cell-cell communication across a tissue-vascular interface.
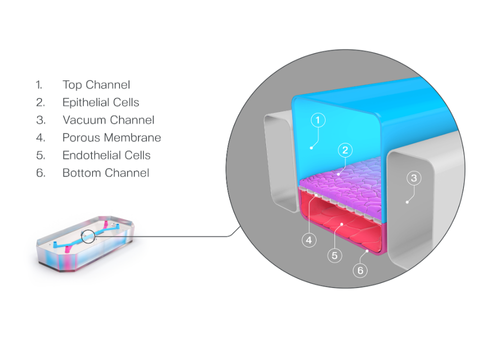
A defining advantage of Organ-Chips is the precise control users have over biomechanical forces. When subjected to media flow and cyclic strain, cells experience the same stresses they would in the body, including intestinal peristalsis, breathing, and vascular flow. Collectively, this organ-specific microenvironment drives more in vivo-relevant gene expression, morphology, and function than standard culture methods, enabling deeper insights into human biology.
In a personalized medicine context, the advantages of Organ-Chips are that they can:
- Use patient-derived samples (stem-cells, primary tumor cells, fibroblasts, endothelial/stromal cells) to create a model specific to that person’s biology.
- Incorporate microenvironmental features (stromal cells, ECM, vascular endothelium, fluid flow, nutrient and gas gradients) that standard culture lacks.
- Deliver drugs under physiologically relevant flow and dosing regimes, better simulating exposure in a patient.
- Allow read-outs of cellular response, toxicities, or disease mechanism with higher fidelity.
- Support functional assays (how the cells behave, not just what mutation they carry), which is increasingly recognized as central to precision medicine.
In short: when you want to make the most of a patient-derived sample (tumor, stem cell, primary tissue), a tumor-in-a-dish or patient-derived organoid may be a start — but an Organ-Chip can be a far better “home away from home” to drive physiologically accurate results.
Below are three case studies illustrating how this plays out across the body, including bone-marrow toxicity, neurodegeneration, and oncology.
Case Study 1
Bone Marrow-on-a-Chip for Drug Toxicity & Patient‐Specific Pathophysiology
Human bone marrow is notoriously difficult to access and study outside the body. Traditional 2D or static 3D culture systems fail to capture the marrow’s complex cellular diversity, mechanical cues, and dynamic exchange between blood and stroma. As a result, predicting myelosuppression from drug exposure or studying patient-specific marrow dysfunction (e.g., in bone marrow failure syndromes) has remained a major challenge.
Organ-on-a-Chip technology provides a physiologically relevant microenvironment that enables direct observation and manipulation of human bone marrow function. Researchers from the Wyss Institute at Harvard University leveraged these advantages to develop a Bone Marrow-Chip as a patient-specific alternative to animal testing for the study of bone marrow pathophysiology:
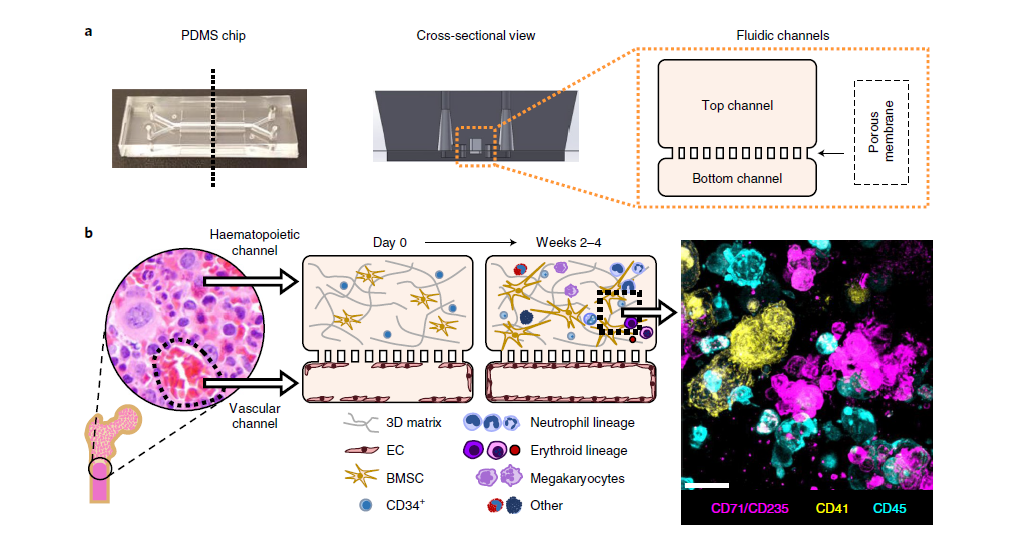
Experimental setup:
- The chip contained a vascular channel lined with human endothelial cells and a parallel channel filled with a fibrin gel seeded with CD34⁺ progenitor and stromal cells.
- Continuous perfusion supported differentiation and maturation of myeloid, erythroid, and megakaryocytic lineages for over four weeks.
- The researchers then exposed the system to clinically relevant chemotherapy doses and radiation, and separately modeled patient-specific bone marrow disorders using cells from individuals with Shwachman-Diamond syndrome.
The Bone Marrow-Chip overcomes long-standing limitations by:
- Recreating bone marrow architecture in vitro: The chip houses hematopoietic progenitor and stromal cells within a 3D extracellular matrix adjacent to a perfused vascular channel lined with endothelial cells. This arrangement mimics the spatial organization of bone marrow sinusoids and the interface between vascular and hematopoietic niches.
- Simulating continuous perfusion: Microfluidic flow delivers nutrients and removes waste—mirroring circulation within the bone marrow. This supports long-term maintenance and differentiation of multiple blood cell lineages, unlike static cultures that quickly lose viability or differentiation potential.
- Providing an in vivo-relevant yet accessible platform: The system combines the physiological complexity of living tissue with the experimental control of an in vitro assay, enabling fine-tuned manipulation of dosing, timing, and microenvironmental factors.
- Supporting the use of patient-derived cells: Researchers could populate the chip with CD34⁺ hematopoietic stem and progenitor cells from both healthy donors and patients with bone marrow failure syndromes—making it a powerful platform for studying patient-specific hematopoietic pathophysiology.
Together, these capabilities create a functional “bone marrow surrogate” that can be experimentally accessed, imaged, and perturbed—something not possible in the human body and rarely achieved in static culture systems.
Major findings & impact:
The Bone Marrow-Chip accurately recapitulated clinical hematologic toxicities, such as lineage-specific depletion after chemotherapy and radiation. Chips seeded with patient-derived cells reproduced hallmark features of Shwachman-Diamond syndrome, including impaired neutrophil maturation.
These results show that the Bone Marrow-Chip can serve as an accessible, human-relevant platform for predicting marrow toxicity, studying disease mechanisms, and testing patient-specific treatment regimens—bridging the gap between in vitro models and human clinical outcomes.
Case Study 2
A Spinal-Cord Organ-Chip Model of Sporadic ALS Using iPSC-Derived Motor Neurons and Blood-Brain-Like Barrier
Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disease characterized by progressive loss of motor neurons. The majority of cases are sporadic, meaning they have no known genetic cause, thus making it difficult to study early disease mechanisms or test personalized treatments. Traditional in vitro models, such as 2D neuron cultures or brain organoids, fall short because they lack the multicellular complexity, vascular interactions, and physiological cues present in the human central nervous system. Moreover, the blood-brain barrier (BBB)—a critical component influencing disease progression and drug delivery—is nearly impossible to replicate in static culture.
Organ-on-a-Chip technology provides a dynamic, multicellular, and perfused platform that bridges this gap between simplified in vitro models and the human nervous system. In this 2025 study published in Cell Stem Cell, researchers from Cedars-Sinai leveraged a microfluidic spinal cord–on–a–chip (SC-Chip) to model sporadic ALS using patient-derived induced pluripotent stem cells (iPSCs).
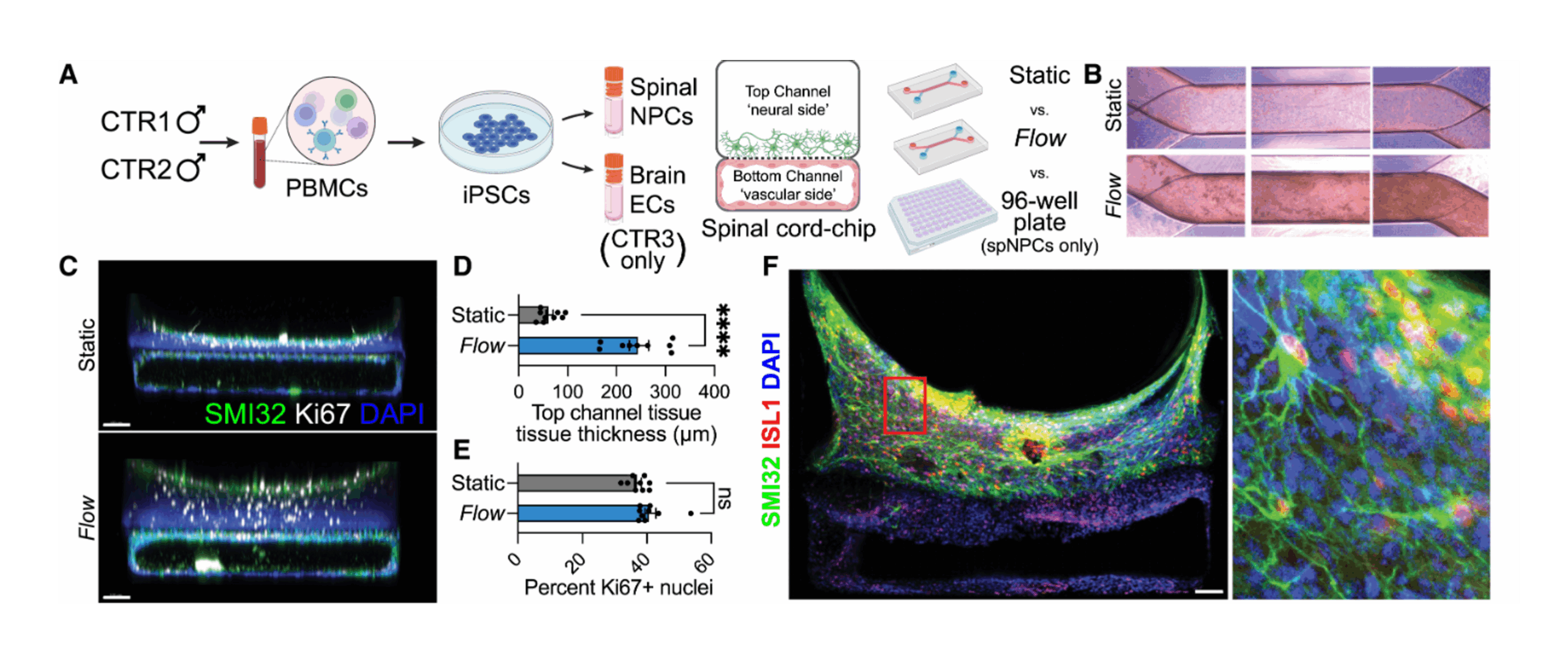
Experimental setup:
- The researchers derived spinal motor neurons from iPSCs obtained from patients with sporadic ALS and healthy controls.
- The vascular channel was seeded with induced brain microvascular endothelial cells (iBMECs) to simulate the blood-brain barrier.
- Over several weeks, the team monitored neuron survival, morphology, and synaptic activity, and conducted bulk and single-cell RNA sequencing to identify disease-associated transcriptional changes.
Using Organ-on-a-Chip technology in this study enabled them to overcome conventional limitations by:
- Recreating the neurovascular unit: The chip featured two adjacent microchannels—one seeded with iPSC-derived spinal motor neurons and the other with brain microvascular endothelial-like cells—separated by a porous membrane. This setup mimics the structural and functional interface between the spinal cord and blood-brain barrier.
- Introducing controlled fluid flow: Continuous microfluidic perfusion supplied nutrients, removed waste, and applied gentle shear stress, promoting maturation of both neuronal and endothelial compartments. This dynamic environment better reproduces physiological conditions than static culture.
- Accommodating patient-derived iPSCs: Using motor neurons reprogrammed from ALS patients’ cells made it possible to model disease-specific phenotypes and compare them directly with healthy control lines.
- Capturing functional cross-talk: The co-culture of spinal cord tissue with a vascular-like channel introduced metabolic and molecular interactions that influence neuronal health—factors missing from most traditional ALS models.
Together, these features created a living, human-relevant “spinal cord microenvironment” that could be accessed and analyzed in unprecedented detail—offering new insight into how ALS develops at the cellular level.
Major findings & impact:
- The perfused SC-Chip supported enhanced maturation and survival of human motor neurons compared to static cultures.
- In chips derived from ALS patient cells, researchers observed early, disease-specific alterations—including disrupted glutamatergic signaling, metabolic dysregulation, and neurofilament accumulation—that were not detectable in traditional culture systems.
- The integrated blood-brain-like barrier exhibited functional permeability properties, enabling exploration of how vascular dysfunction contributes to ALS pathology and how it might affect drug penetration.
By combining patient-derived neurons with a physiologically active barrier under flow, the SC-Chip revealed patient-specific disease mechanisms and potential therapeutic targets previously masked in simpler systems.
This study demonstrates that Organ-on-a-Chip technology can serve as a window into complex, patient-specific neurodegenerative processes—offering an accessible yet physiologically authentic platform for mechanistic discovery and, ultimately, personalized drug testing in ALS.
Case Study 3
Patient-Derived Esophageal Adenocarcinoma Organ-Chips for Functional Precision Oncology
Esophageal adenocarcinoma (EAC) is one of the most aggressive cancers of the digestive tract, with limited treatment options and highly variable responses to chemotherapy. In clinical practice, many patients undergo neoadjuvant chemotherapy (NACT) before surgery, but up to half fail to respond—exposing them to unnecessary toxicity and delays in curative treatment.
Traditional ex vivo models, such as patient-derived organoids (PDOs), provide valuable insights into tumor biology but often lack the dynamic tumor microenvironment (TME) features that govern therapeutic response—such as stromal interactions, nutrient gradients, and fluid flow. Without these elements, static models cannot reliably predict how a patient’s tumor will behave in the body.
Organ-on-a-Chip technology offers a living, perfused model of the tumor microenvironment that maintains patient-specific biology while introducing physiologically relevant mechanical and chemical cues. In a 2025 study published in the Journal of Translational Medicine, researchers at McGill University and the Wyss Institute created an Esophageal Adenocarcinoma (EAC) Organ-Chip (EAC-Chip):
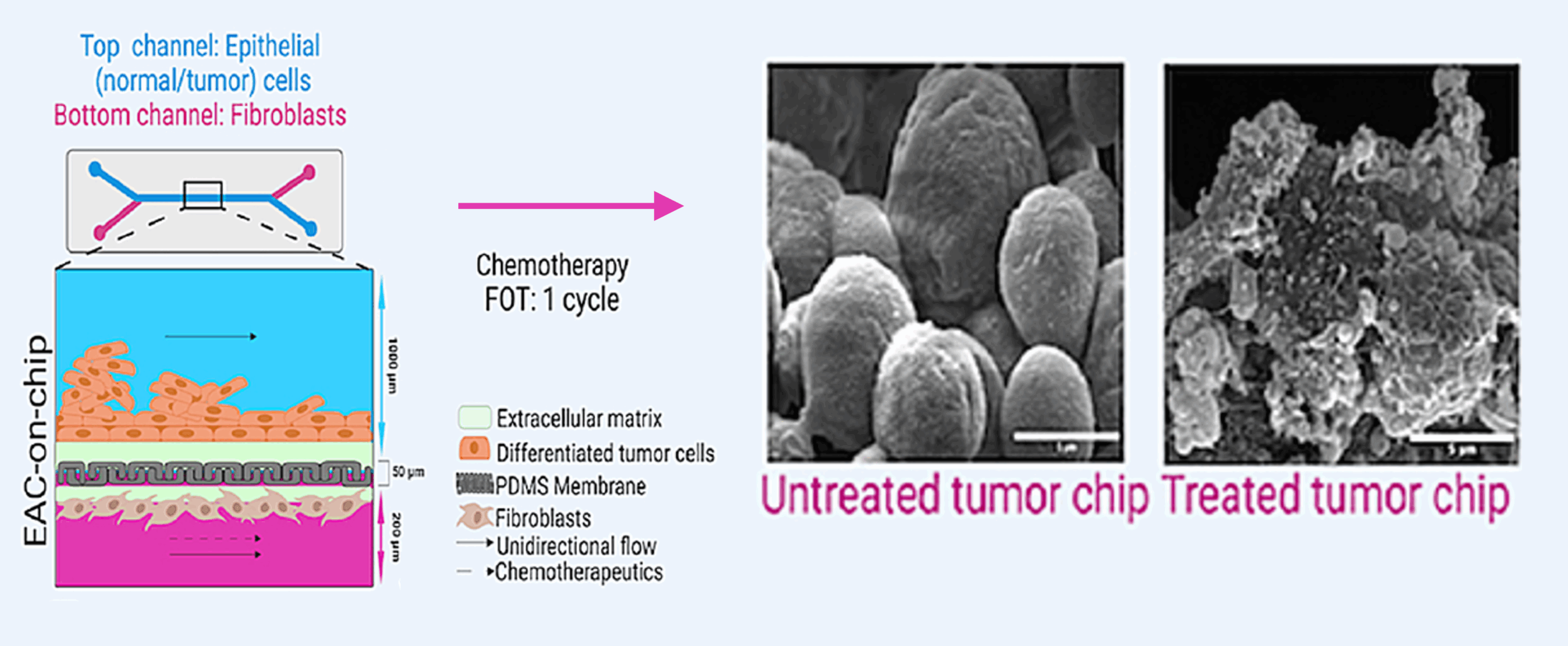
Experimental setup:
- Tumor biopsies and matched stromal fibroblasts were isolated from eight patients with treatment-naïve EAC. The tumor organoids were cultured in one channel of the microfluidic device, while patient-derived fibroblasts populated the opposing channel.
- The same neoadjuvant chemotherapy regimen used in each patient’s clinical care was perfused through the system at physiologically relevant concentrations.
- Researchers evaluated viability, histology, and biomarker release to assess drug sensitivity, with results available within 2-3 months following diagnostic biopsy—well within NACT timelines.
The EAC-Chip overcame key challenges of functional precision oncology by:
- Recreating the tumor–stroma interface: The chip contained two adjacent microchannels—one seeded with tumor organoids derived from patient biopsies and the other with matched cancer-associated fibroblasts—separated by a porous membrane that allowed bidirectional signaling. This setup recreates the communication between cancer and stromal compartments that strongly influences therapy response.
- Supporting use of patient-derived samples: Each chip was seeded with tumor and fibroblast cells obtained directly from individual patients, preserving genetic heterogeneity and enabling patient-specific response prediction.
- Simulating clinically relevant drug exposure: The microfluidic perfusion system allowed researchers to deliver chemotherapy drugs under time-dependent flow conditions that mirror intravenous (IV) administration in patients. This means that the concentration and exposure pattern of the drugs in the chip followed the same pharmacokinetic profile a patient would experience during neoadjuvant chemotherapy. As a result, cells within the chip received clinically relevant dosing regimens—something static organoid cultures cannot replicate.
- Providing a clinically relevant yet accessible system: Because the assay and associated analysis can be completed in 2-3 months from the time of the diagnostic biopsy, results can align with patient treatment timelines—bridging the gap between laboratory modeling and actionable clinical decisions.
Together, these design features make the EAC-Chip a functional and accessible “tumor-on-a-chip” that mirrors both the structure and drug-response behavior of human cancers.
Major findings & impact:
- The EAC-Chip faithfully preserved tumor architecture and genetic fidelity, maintaining hallmark features of each patient’s cancer.
- Remarkably, the chip predicted individual patient responses to chemotherapy with 100% concordance: tumors that responded in the clinic also showed significant cell death in the chip, while resistant tumors remained viable.
- The perfused chip system captured tumor–stroma interactions and microenvironmental effects that static organoid cultures failed to replicate, making it more predictive of clinical outcomes.
- The ability to model response and gain actionable insights in 2-3 months demonstrates that Organ-Chip assays could realistically fit into the therapeutic workflow—allowing oncologists to refine or replace treatment plans before committing patients to ineffective regimens.
- Beyond EAC, this approach establishes a template for functional precision oncology, where each patient’s tumor can be tested in a physiologically relevant environment to guide individualized therapy.
In essence, the Esophageal Adenocarcinoma Organ-Chip transforms patient-derived samples into a living diagnostic tool—one that combines the fidelity of patient-specific tumor cells with the physiological realism of microfluidic perfusion. It’s a clear demonstration of how Organ-on-a-Chip technology can make the most of limited patient samples to deliver actionable insights that genomics alone cannot provide.
What These Examples Teach Us About Personalized Medicine
Together, the above case studies highlight several key themes for personalized medicine enabled by Organ-on-a-Chip technology:
- Patient-derived samples matter: Whether CD34⁺ marrow progenitors, iPSC motor neurons, or tumor organoids and fibroblasts — using cells from the actual patient brings the model closer to reality.
- Microenvironment and flow matter: Each study shows that recreating stromal/endothelial compartments, fluid perfusion, and physiological architecture makes a difference in predictive fidelity and mechanistic insight.
- Functional assays (not just genomics) matter: These platforms go beyond just reading genetic mutations — they assess how the cells behave in a physiological context, a critical step for precision medicine.
- Clinical-timescale results: The Bone Marrow-Chip matured cells over 4 weeks; the ALS SC-Chip achieved matured phenotypes earlier than static culture; the EAC-Chip delivered actionable insights in ~2 months from the time of diagnostic biopsy — all moves toward actionable timelines in a clinical or translational context.
- Broad disease applicability: The three examples span hematology/toxicology, neurodegeneration and oncology — showing how Organ-Chips are broadly useful for personalized medicine, not just cancer.
Future Outlook
The convergence of personalized medicine and Organ-on-a-Chip technology offers a powerful pathway forward. As Organ-Chips platforms become more standardized, automated, and integrated with patient-derived samples and clinical workflows, we may eventually see them function as routine companion diagnostic tools, preclinical drug testing platforms, or even clinical decision support systems.
For practitioners, researchers and clinicians interested in precision medicine, Organ-on-a-Chip technology offers an exciting opportunity: to make the most of patient-derived samples, to ask functional questions in physiologically-relevant systems, and to bring actionable model-based predictions closer to the bedside.
Conclusion
Personalized medicine demands models that reflect patient biology — not just genomics, but how those cells live, interact and respond under real-world conditions. Organ-Chip platforms provide that model: an in vivo-relevant milieu for patient-derived samples that preserves complexity, recreates organ-specific microenvironments, and allows functional read-outs. From modeling bone marrow toxicities, to uncovering neurodegenerative mechanisms in ALS, to predicting chemotherapy response in esophageal cancer — the promise of Organ-Chips in personalized medicine is already being realized. As the technology continues to mature and become integrated into translational workflows, we come closer to a future where every patient’s sample can be placed on a chip — and the best therapy chosen, before treatment begins.
Interested in learning more? Register for our upcoming webinar to hear directly from the senior authors of the EAC-Chip study on how they used Organ-Chips to improve functional precision oncology.





