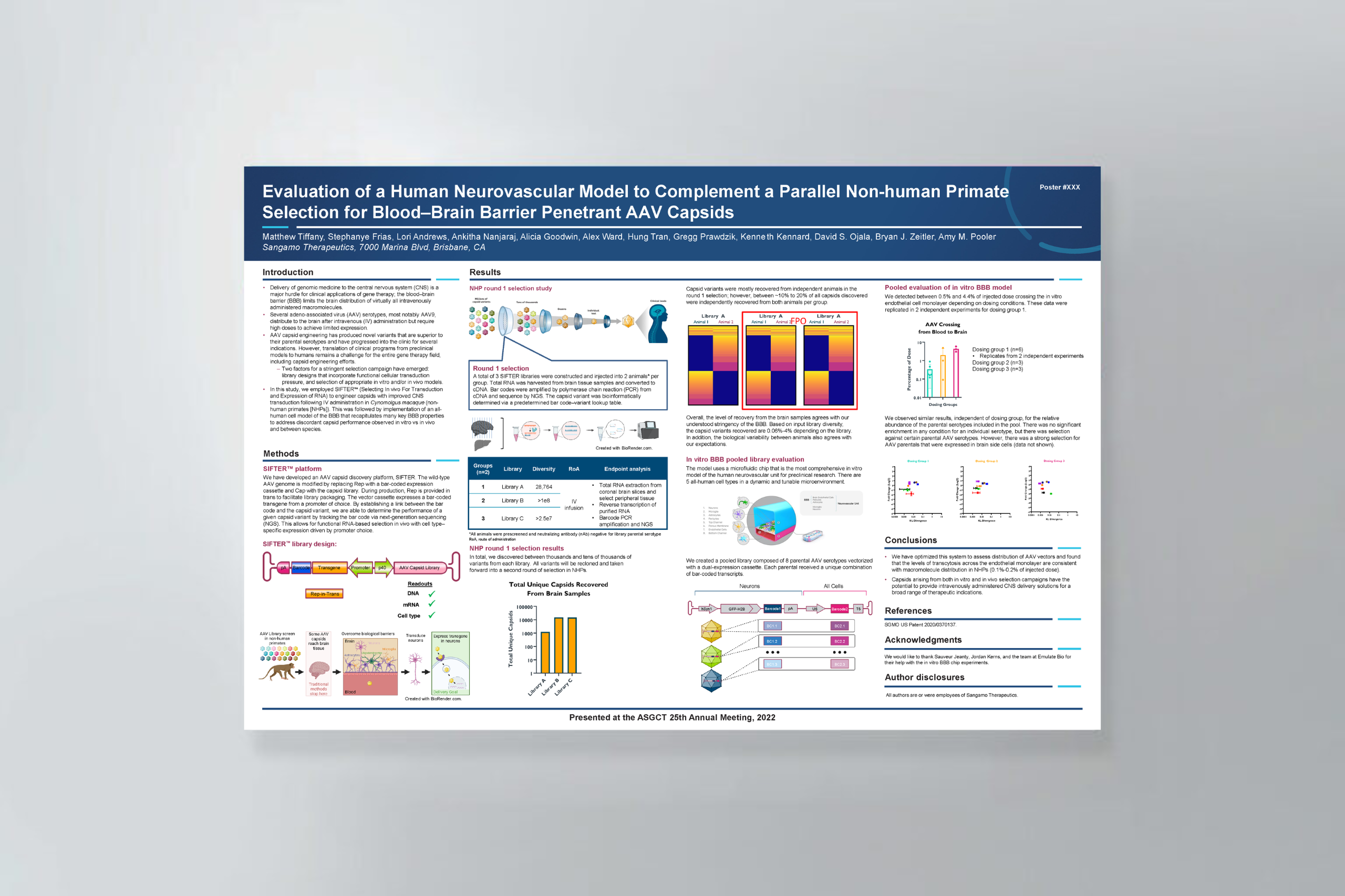
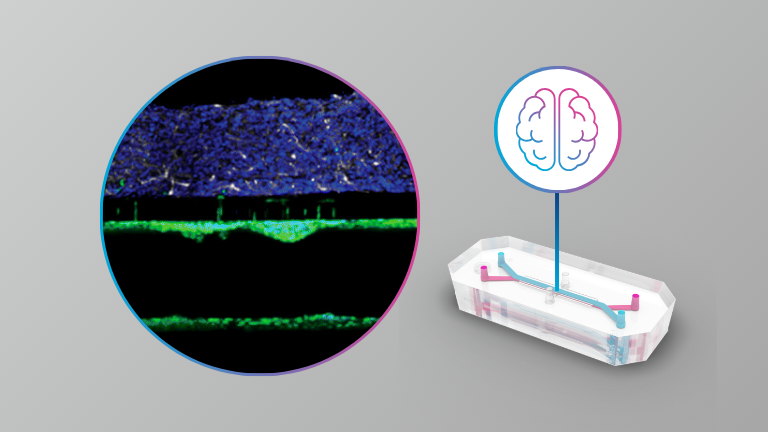
Organ Model: Brain
Application: Neuroscience
Abstract: While cells in the human body function in an environment where the blood supply constantly delivers nutrients and removes waste, cells in conventional tissue culture well platforms are grown with a static pool of media above them and often lack maturity, limiting their utility to study cell biology in health and disease. In contrast, organ-chip microfluidic systems allow the growth of cells under constant flow, more akin to the in vivo situation. Here, we differentiated human induced pluripotent stem cells into dopamine neurons and assessed cellular properties in conventional multi-well cultures and organ-chips. We show that organ-chip cultures, compared to multi-well cultures, provide an overall greater proportion and homogeneity of dopaminergic neurons as well as increased levels of maturation markers. These organ-chips are an ideal platform to study mature dopamine neurons to better understand their biology in health and ultimately in neurological disorders.