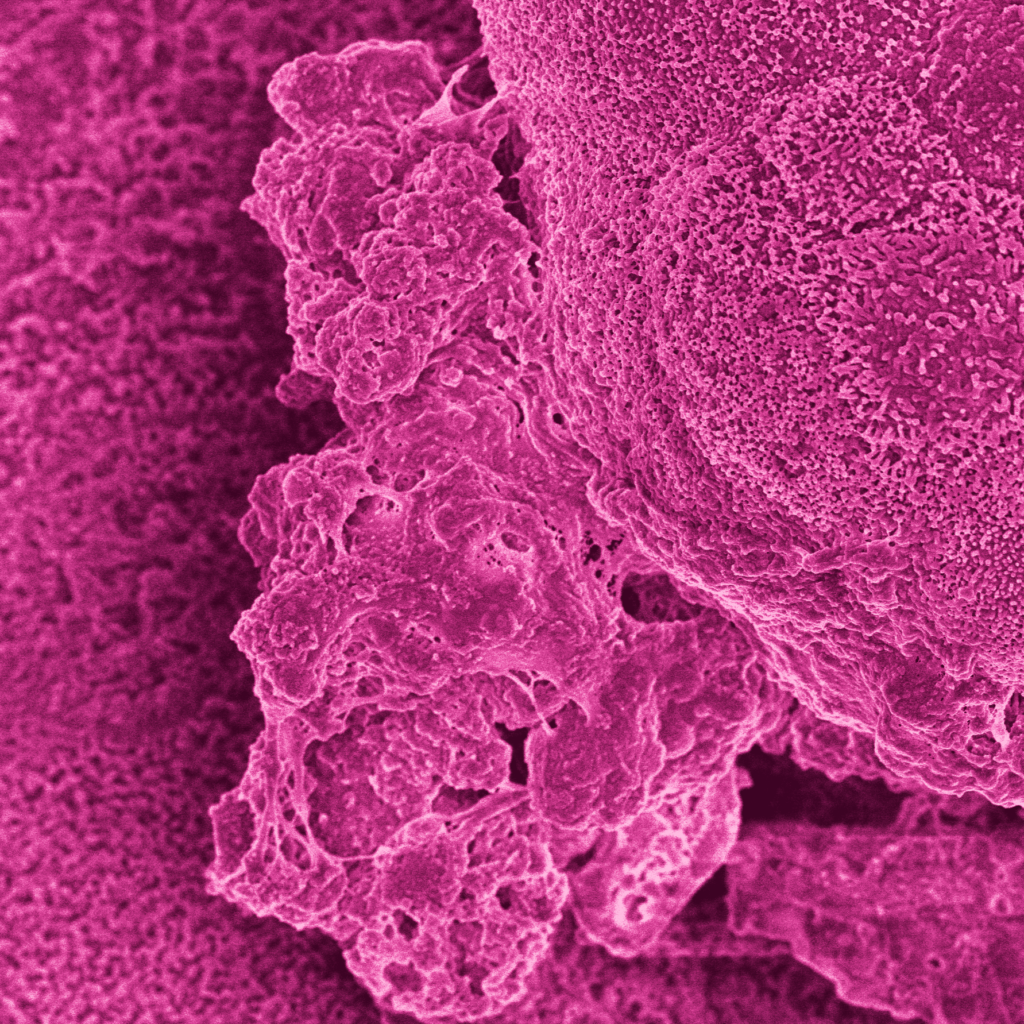
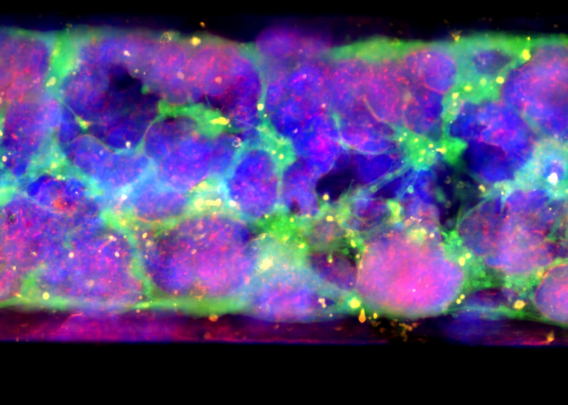
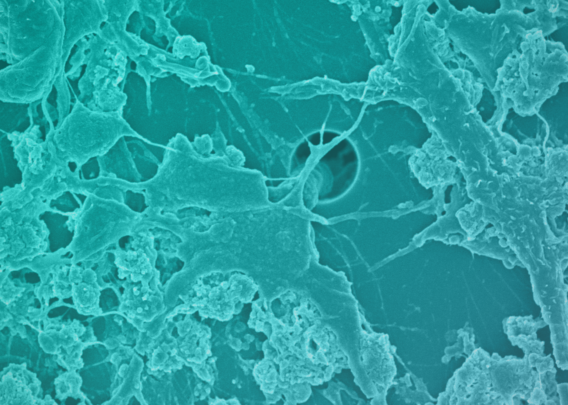
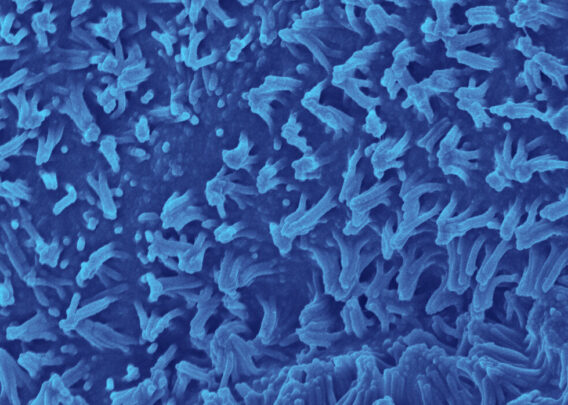
See how the Colon Intestine-Chip has been used to model cytokine-mediated intestine inflammation and barrier disruption.
Bacteria, viruses, and potential toxins all transit through the human intestines. In healthy conditions, the intestinal barrier serves as a protective wall, helping to prevent the engagement of these would-be biological agents. However, when this protective barrier breaks down, problems arise. Intestinal barrier dysfunction is often associated with chronic inflammation and is increasingly linked to pathological conditions ranging from inflammatory bowel disease (IBD) to Parkinson’s Disease. These observations suggest that intestinal barrier deterioration may influence pathogenesis of some diseases and have value as a therapeutic target.
Understanding of the processes that lead to intestinal barrier deterioration is limited, due in part to a lack of human-relevant models. The intestine is a dynamic organ consisting of many diverse cell types whose behaviors are influenced by the complex milieu of cell-cell interactions, peristaltic contractions, and various environmental factors. Normally, it is exceedingly difficult for single-model systems to capture this complexity. However, recent evidence suggests Organ–a-Chip technology can provide a strong approximation of in vivo conditions, making it an invaluable tool for studying intestinal biology.
In a paper published in Cellular and Molecular Gastroenterology and Hepatology, researchers from Emulate characterize a colon intestine model in which patient-derived colonic organoids are cultured in a dynamic Organ-on-a-Chip platform. Unlike conventional models, this “gut-on-a-chip” includes primary human cells that are subject to biomechanical forces and co-cultured with intestine-specific endothelial cells, closely resembling the phenotypic characteristics of in vivo tissue.
Collectively, the data presented in this paper highlights the Colon Intestine-Chip’s ability to provide detailed insights into the human intestine barrier in health and disease settings.
Experimental Overview
Research Area: Gastroenterology, Disease pathology
Organisms: Human
Sample Types: Colon Intestine-Chip
Research Question: Can this “gut-on-a-chip” be used to model the effects of cytokines, therapeutics, and other agents on intestinal barrier integrity?
Results:
- Co-culture of colonoids with endothelial cells in the Colon Intestine-Chip results in improved epithelial cell phenotypic and transcriptomic profiles that more accurately represent in vivo observations compared to immortalized epithelial cell monolayers or colonoids cultured in suspension.
- Perfusion of the Colon Intestine-Chip vascular chamber with IFN-γ promotes inflammatory phenotypes in epithelial cells, breakdown of tight junctions in the epithelial cell barrier, and subsequent increased barrier permeability.
- Treatment of the Colon Intestine-Chip with Interleukin-22 (IL-22) promotes inflammatory signaling and tight junction breakdown, shedding light on the potential role of IL-22 in the pathogenesis of intestinal barrier deterioration.
Conclusion: The Colon Intestine-Chip represents an improved model of the human colon that contains a heterogeneous epithelial cell layer displaying phenotypic and transcriptomic profiles similar to those observed in vivo. Using this model, researchers can effectively investigate the mechanisms behind cytokine-mediated inflammation and the efficacy of therapeutic candidates on human colonic barrier integrity. Because of this, the Colon Intestine-Chip can help shed light on the complex relationship between intestinal barrier integrity and disease pathogenesis.
Modeling a dynamic organ
Researchers aiming to study gastrointestinal physiology and disease primarily rely on three types of models: animals, organoids, and conventional monolayer cultures of immortalized cell-lines. Each of these has been invaluable in advancing our understanding of gut physiology; however, none are able to recreate the critical features of the human intestine that influence cellular response to stressors and—in turn—disease pathogenesis. Because of this, it has been challenging to translate results from these models into effective disease modifying therapies.
Organ-on-a-Chip technology presents a promising alternative. Emulate Organ-Chips are three-dimensional, dynamic systems that co-culture tissue-specific cell types—such as epithelial cells and immune cells—alongside endothelial cells under fluid flow and in the presence of tissue-specific extracellular matrix proteins. Selectively seeding cells into the chip’s two channels enables the formation of a vascular chamber consisting of endothelial cells and a tissue chamber containing the remaining cell types. These two channels are separated by a thin, porous membrane to enable communication between cell chambers while maintaining distinct microenvironments.
To improve on current models of the intestines, researchers from Emulate leveraged Organ-Chip technology to develop a Colon Intestine-Chip.
Colon Intestine-Chip: an improved model of gastrointestinal physiology
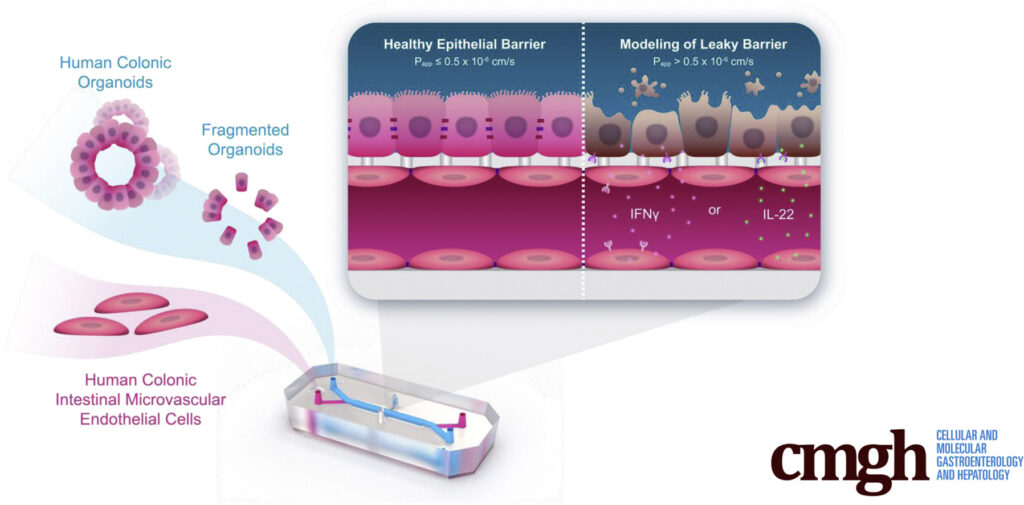
To create the Colon Intestine-Chip, Apostolou et al., made use of Emulate’s Organ-on-a-Chip technology, which enables multiple cell types to be co-cultured in a dynamic environment. Colonic organoids (colonoids), which came from healthy patient biopsies and were mechanically dissociated, served as the basis for the chip’s intestinal chamber. In parallel, colonic human intestinal microvascular endothelial cells were seeded in the vascular chamber. When exposed to unidirectional media flow as well as cyclic 10% stretch to emulate peristalsis, the model closely resembles the microenvironment intestinal cells would experience in vivo.
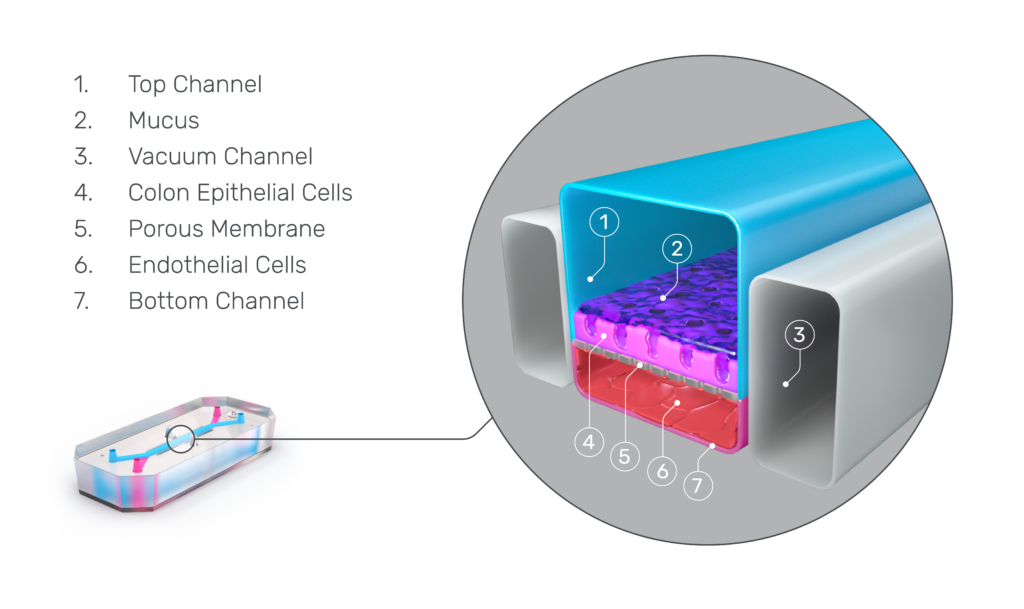
Characterization of the Colon Intestine-Chips revealed a close phenotypic resemblance to healthy colonic barriers, including: The formation of a confluent, highly polarized epithelial cell barrier with low permeability (0.89 x 10-6 cm / s); the localization of tight junction proteins at intercellular junctions; the asymmetric distribution of ion channels; and the formation of a mature brush border with densely packed microvilli.
Notably, the epithelial cells’ mature phenotype was dependent on the presence of endothelial cells within the chip’s vascular chamber. Exclusion of endothelial cells from the Colon Intestine-Chip led to increased barrier permeability, decreased tight junction formation, and decreased epithelial cell polarization, collectively demonstrating the importance of endothelial co-culture with epithelial cells in modeling the intestinal barrier.
Given these findings, it’s unlikely that colonoids or conventional monolayer culture models could mimic in vivo conditions as well as the Colon Intestine-Chips. This claim is reinforced by transcriptomic data collected from Colon Intestine-Chips and colonoid cells grown in suspension, which showed that the presence of endothelial cells and periodic stretching in Colon Intestine-Chips produced superior gene expression profiles.
Using the Colon Intestine-Chip to model gut barrier breakdown
To study To study the pathophysiology of gut barrier dysfunction, the team perfused the vascular chamber with interferon gamma (IFN-γ), a cytokine known to affect the pathogenesis of inflammatory bowel disease.
Within two days of treatment, the epithelial cell barrier showed clear signs of distress. Epithelial barrier permeability increased in an IFN-γ-concentration-dependent manner, tight junction proteins were sequestered to the cellular cytoplasm, and F-actin staining revealed cell deformations and poorly defined cell borders. Collectively, these findings indicate that the presence of IFN-γ was driving a breakdown in the epithelial cell barrier—a conclusion that was strongly reinforced by an increase in epithelial cell death (as indicated by elevated levels of cleaved caspase).
What’s more, treatment with IFN-γ prompted an increase in the cytokine IL-6 and vascular adhesion molecule-1—both of which have been found in the sera of patients with inflammatory bowel disease—reinforcing that this model is able to accurately recreate aspects of an IBD-like clinical phenotype.
Advancing our understanding of gut barrier physiology and pathophysiology
Interleukin-22 (IL-22) is a cytokine released by various immune cells in response to pathogens. To date, our understanding of IL-22’s role in intestinal health and disease is incomplete. Various ulcerative colitis studies using mice have found conflicting results, with some results suggesting IL-22 has pro-inflammatory effects and others suggesting it has anti-inflammatory effects.
After showing that the Colon Intestine-Chip model can accurately recreate a mature intestinal epithelial cell barrier phenotype and model the effect of well-characterized, barrier-disrupting cytokine IFN-γ, the authors evaluated whether the model could shed light on the true role of IL-22 in intestinal barrier function.
Before administering IL-22, the team first confirmed the expression of the IL-22 receptor and found that expression was higher in the Colon Intestine-Chip compared with organoids in suspension. These results suggest that our incomplete understanding of IL-22’s role in barrier homeostasis may be due in part to limited gene expression in other models.
Perfusing IL-22 through the vascular chamber negatively affected epithelial cell barrier function in the Colon Intestine-Chip model. Barrier permeability increased, cell morphology became aberrant, and transcriptomic profiles and immunofluorescent staining revealed a marked increase in apoptosis. Taken together, these results suggest that IL-22 drives barrier dysfunction.
Conclusion
ln total, this study showed that the Colon Intestine-Chip can be a powerful model for studying intestinal barrier dysfunction. Immunofluorescent staining, scanning electron microscopy, and RNA sequencing data show that the Colon Intestine-Chip model produces a mature epithelial cell phenotype that responds to inflammatory cytokines, such as IFN-γ, in ways that reflect observations from patients with inflammatory bowel disease.
Importantly, this study showed that endothelial co-culture is critical to promote a mature, functional epithelial phenotype, driving positive effects on cell morphology, polarization, and barrier formation. This insight highlights an advantage Organ-Chips have over intestinal models without endothelial co-culture, such as organoids in suspension.
The team’s use of the Colon Intestine-Chip to study IL-22’s effects on intestinal barrier integrity demonstrates the potential to apply this model in studying mechanisms of intestinal barrier dysfunction. Collectively, this study shows how the Colon Intestine-Chip is a more physiologically relevant model of the human colon that researchers can use to study gastrointestinal disease pathogenesis and the efficacy or safety of preclinical drug candidates in preclinical stages.