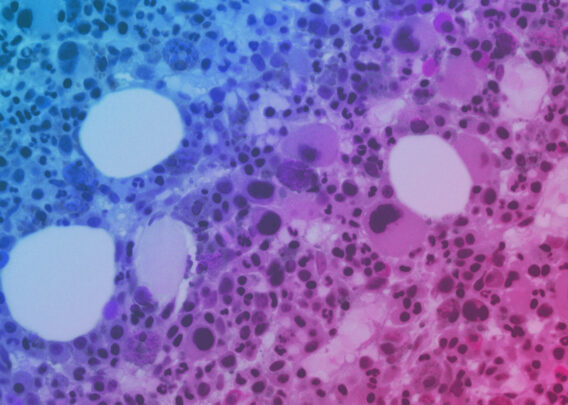
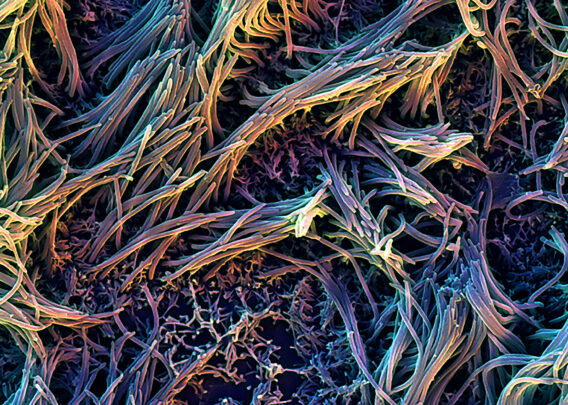
2024 was filled with groundbreaking discoveries that showcase the transformative potential of Organ-on-a-Chip technology. From uncovering new disease mechanisms to advancing drug safety and efficacy testing, these studies highlight the various way that Organ-Chips are contributing to cutting-edge research. Let’s take a closer look at ten of the most impactful Organ-Chip publications of 2024.
1. Unlocking the Role of the Lung Glycocalyx in Bacterial Pneumonia
The lungs’ glycocalyx, a thin protective layer, plays a vital role in shielding against infections. Researchers at Universitätsmedizin Berlin leveraged the Alveolus Lung-Chip to investigate how glycocalyx breakdown contributes to bacterial pneumonia caused by Streptococcus pneumoniae. Their findings revealed that enzymatic degradation of glycocalyx components, such as hyaluronan and heparan sulfate, exacerbates bacterial load, inflammation, and tissue damage.
The study also highlighted potential therapeutic targets to preserve glycocalyx integrity, which could mitigate lung injury and prevent systemic disease progression. Using the Lung-Chip, the team replicated the human alveolar-capillary interface in a way that traditional animal models and static cell cultures could not. These insights offer a new perspective on respiratory health, laying the groundwork for therapies that could revolutionize pneumonia treatment.

2. Mitigating Radiation Injury
Radiation-induced liver injury (RILI) is a significant side effect of radiotherapy and radiation exposure, with few effective prevention strategies. Traditional models often fail to mimic the complexity of human liver physiology, limiting the ability to predict clinical outcomes. The Emulate Liver-Chip’s co-culture system, combined with continuous perfusion, provided a closer approximation of in vivo conditions.
The NIH utilized the Liver-Chip to study the human-specific effects of radiation, focusing on the interaction between hepatocytes and sinusoidal endothelial cells. This dynamic model revealed key biomarkers associated with radiation injury, including inflammation and fibrosis pathways. This research holds promise for improving cancer treatment safety and preparing for radiation-related emergencies, offering a human-relevant tool for developing mitigation strategies.
3. A New Approach to Organ Preservation with Biostasis Drugs
Imagine a world where organs for transplantation remain viable for extended periods. Researchers have made strides toward this vision with SNC80, a biostasis drug that slows metabolism without cooling tissues. Using Gut-Chips and Liver-Chips, the team demonstrated how SNC80 reduces oxygen consumption and maintains tissue health, even under stress conditions similar to those during transplantation.
What sets SNC80 apart is its reversibility—once the drug is removed, tissues return to normal metabolic activity without adverse effects. This research could pave the way for preserving organs longer and enabling life-saving procedures in remote or resource-limited settings. It’s a powerful example of how Organ-on-a-Chip technology can facilitate breakthroughs in critical care.
4. New Insights on Cancer Therapeutic-Induced Cardiotoxicity Using a Multi-Lineage Human Heart-Chip
Cancer drugs like sorafenib are life–saving but can have harmful side effects on the heart that are difficult to predict in preclinical models. To address this, Cedars-Sinai researchers used a Heart-Chip model that mimics the human cardiovascular environment by integrating cardiomyocytes and vascular endothelial cells. The study uncovered how sorafenib affects both cell types, leading to reduced contractility and vascular dysfunction.
What makes this research remarkable is the chip’s ability to replicate human-specific responses, surpassing the limitations of animal models. The Heart-Chip provides a scalable, precise platform for evaluating cardiotoxicity in drug candidates, enabling safer cancer treatments without compromising efficacy. This innovation is a step toward more patient-centered care in oncology.
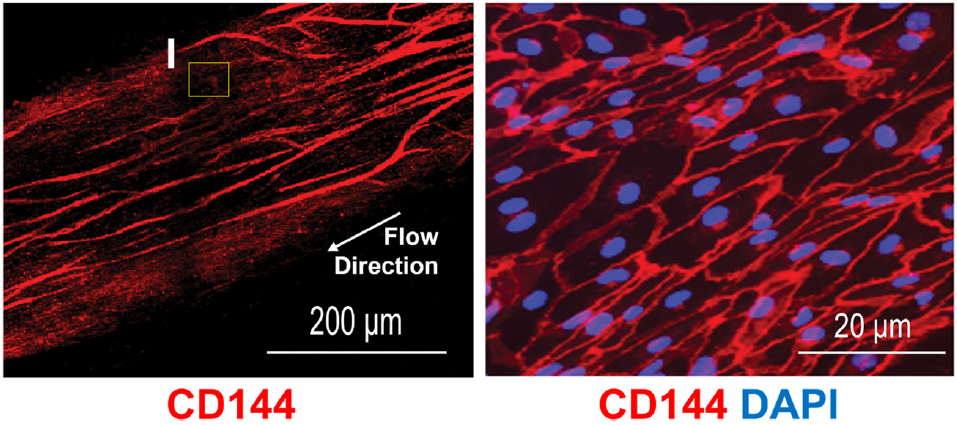
5. Breaking New Ground in Intestinal Barrier Modeling with the hiPSC-Derived Intestine-Chip
The small intestine is a marvel of complexity, and replicating its environment has long been a challenge. Researchers at the University of Groningen tackled this with a hiPSC-derived Intestine-Chip, which mimics the gut’s crypt-villus structure and multi-lineage cell composition. This Organ-Chip model offers an unprecedented look at gut health, including nutrient absorption and immune interactions.
By integrating dynamic conditions such as flow and growth factor gradients, the Intestine-Chip enables drug testing and disease modeling with unparalleled accuracy. This platform is not just a tool for research but a gateway to developing personalized treatments for gastrointestinal disorders.
6. Exploring the Intersection of Cannabidiol and Liver Health Through a Liver-Chip S1 Quad-Culture Model
With the rise of CBD-based products, understanding their safety profile is essential. Using the Liver-Chip S1 Quad-Culture, researchers examined how high doses of cannabidiol affect liver health. They found that while therapeutic doses were generally safe, higher concentrations disrupted antioxidant pathways and triggered mild liver injury.
The study highlights the importance of using human-relevant models to evaluate drug safety. The Liver-Chip’s ability to replicate complex liver functions provides a predictive tool for assessing the risks of CBD and other cannabinoids, ensuring safer consumer products.
7. Comparing Gut-on-a-Chip and Transwell Models for Peptide Formulations
Testing oral drug formulations has long relied on models like Transwell systems, but these often fail to replicate the complexities of human intestinal physiology. To address this gap, researchers from Merck developed a Gut-Chip, a dynamic platform that mimics the structure and function of the human intestine with features like continuous flow and 3D villus-like structures. Their study demonstrated that the Gut-Chip provides more accurate predictions of intestinal absorption and drug permeability compared to traditional methods.
What sets this research apart is the Gut-Chip’s ability to simulate in vivo-like conditions, reducing the reliance on animal models while enhancing translational success. This enables a more reliable preclinical assessment of oral drugs, helping pharmaceutical companies develop safer and more effective treatments.
8. Advancing Vaccine Testing with Organ-on-a-Chip Technology
Understanding human immune responses to vaccines has been a longstanding challenge, often relying on animal models that fail to capture human-specific dynamics. Researchers at Institut Pasteur tackled this issue by developing a Lymphoid Organ-Chip, which enabled them to study memory B-cell activation and antibody production in response to mRNA vaccines. The platform revealed critical insights into how immune imprinting impacts booster efficacy for evolving viral strains.
The Lymphoid Organ-Chip’s ability to replicate human immune responses using a minimal number of cells sets it apart from conventional models. This innovative tool is transforming vaccine development, offering faster, more accurate testing methods to improve pandemic preparedness and public health outcomes.
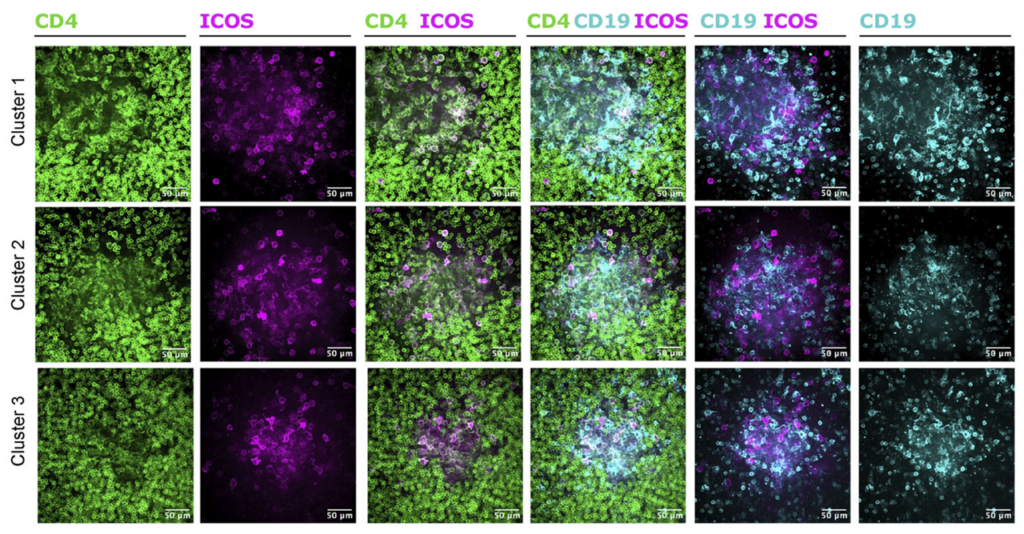
9. Progressing Women’s Health with Cervix Organ-Chips
Reproductive health research has often been limited by the lack of models that capture the unique physiology of the cervix. To overcome this, researchers at the Wyss Institute developed the Cervix-Chip, a platform designed to replicate the cervical environment, including mucus production and host-microbiome interactions. Their work explored how healthy and dysbiotic bacterial communities affect cervical barrier function and immune responses.
The Cervix-Chip’s ability to model hormone sensitivity and microbial dynamics offers a new lens for studying conditions like bacterial vaginosis and improving reproductive health. This platform could enable researchers to develop more effective therapies in a critical but under-researched area of women’s health.
10. Shaping the Future of Brain Disease Research
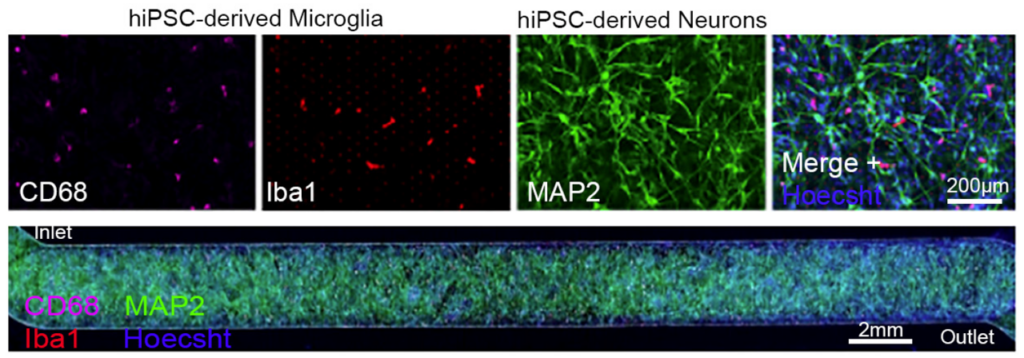
Predicting which drugs will cross the blood-brain barrier (BBB) presents one of the biggest challenges in developing drugs for neurological diseases. Researchers at Regeneron used the Brain-Chip, a platform combining key brain cell types with advanced microfluidics, to explore how therapeutics interact with the BBB. Their findings demonstrated the chip’s ability to model functional neuronal circuits, neuroinflammation, and the transport of drugs across the barrier.
This breakthrough lies in the Brain-Chip’s capacity to mimic human-specific BBB dynamics, surpassing the limitations of animal models. The platform is paving the way for innovative treatments for Alzheimer’s, Parkinson’s, and other neurological conditions, revolutionizing the future of brain health research.
These publications were featured on our LinkedIn page in December of 2024 to celebrate reaching 25,000 subscribers. To stay up to date with the latest Organ-Chip research, news, and advancements, be sure to follow us on LinkedIn and Twitter!
Want to explore Organ-Chip publications further? View our publications database here.