Inflammatory bowel disease (IBD) is increasing around the world. In 1990, around 3.7 million people had the condition; by 2017, that number had increased to 6.8 million. Nearly half of IBD patients don’t respond to current treatments, and even for the lucky ones, therapeutic efficacy can wane over time. As a result, there is an urgent need to develop a new generation of IBD therapies.
Unfortunately, ineffective drug development models are hampering the search for more effective treatments. Conventional two-dimensional (2D) cell models only capture bits and pieces of IBD’s complexity, and many three-dimensional (3D) culture models like organoids fall short because they lack critical biological features, such as vasculature and biomechanical forces.
Animal models have their own drawbacks, as their immune systems fail to replicate many of the mechanisms associated with human immunity.
“If you look at the physiology of cardiac muscle or neurons between humans and mice, they’re fairly similar,” said Christopher Carman, PhD, Director of Immunology at Emulate. “There’s more divergence in immunology, and it can be really challenging to extract meaningful insights around immune-system-driven mechanisms. That’s why so many therapeutics fail.”
To remedy this, Emulate has developed a Colon Intestine-Chip that combines primary human tissue, vasculature, mechanical forces, and (most importantly) immune cell recruitment to recapitulate the biology that drives IBD.
Understanding How IBD Evolves
IBD begins with an unknown tissue insult, and the body responds by producing inflammatory cytokines and chemokines. In turn, these proteins recruit immune cells to the intestine, inducing further inflammation.
This process generates a cytokine cascade. Two proteins in particular, interferon gamma (IFNγ) and IL-22, act directly on colon epithelial cells, driving cell death, microvilli loss, and destruction of the tight junctions that guard intestinal permeability.
“That is a critical hallmark of this disease,” said Carman. “As a result, intestinal material, including bacteria and bacterial products, leak into the interstitial space, driving even more inflammation.”
Making the Colon Intestine-Chip
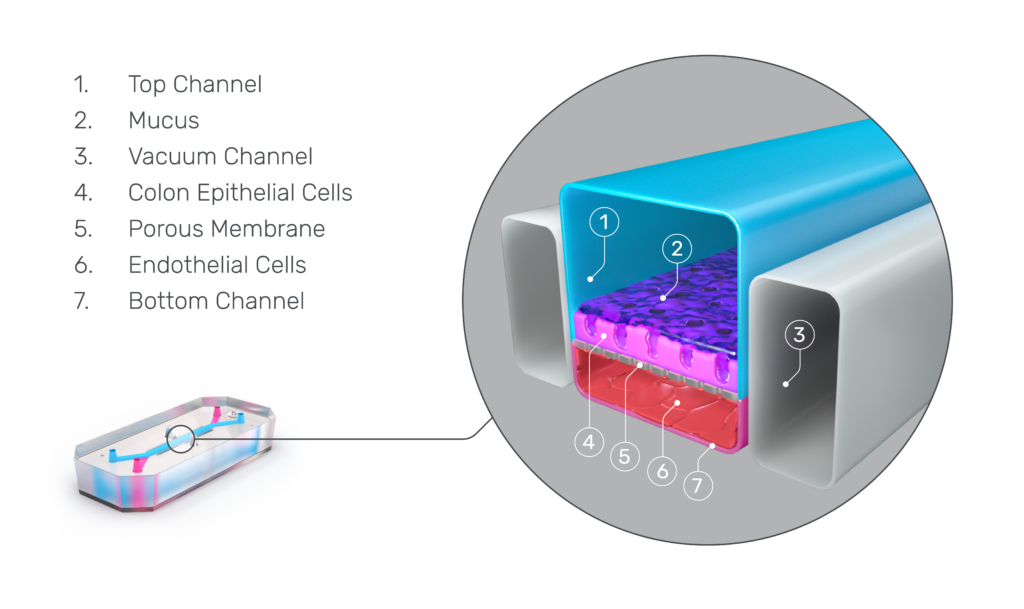
The Emulate Colon Intestine-Chip was designed to precisely recapitulate this inflammatory cascade.
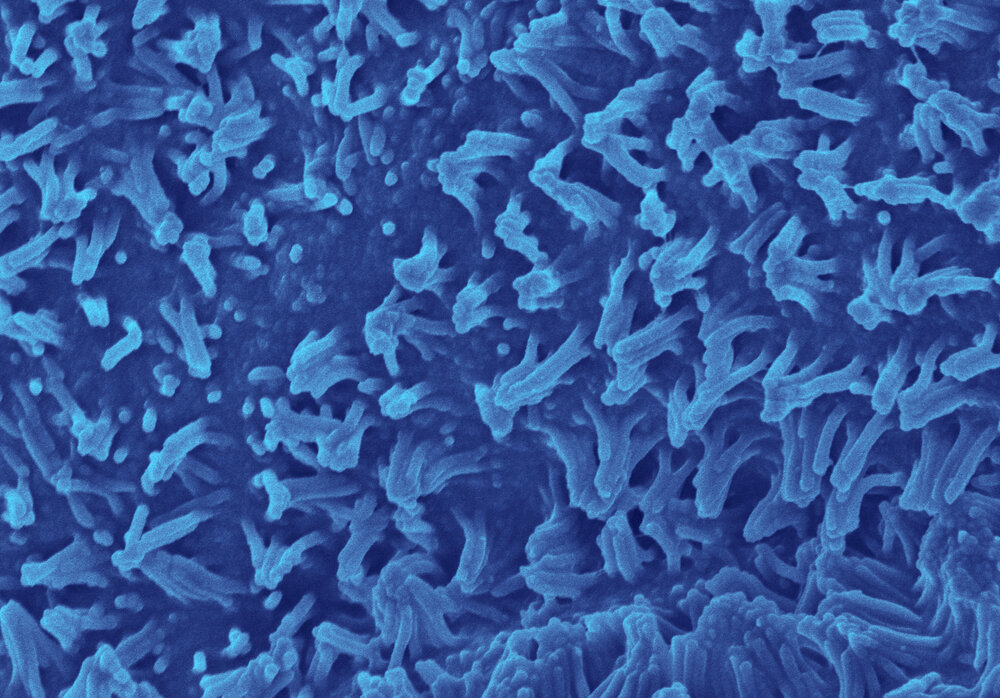
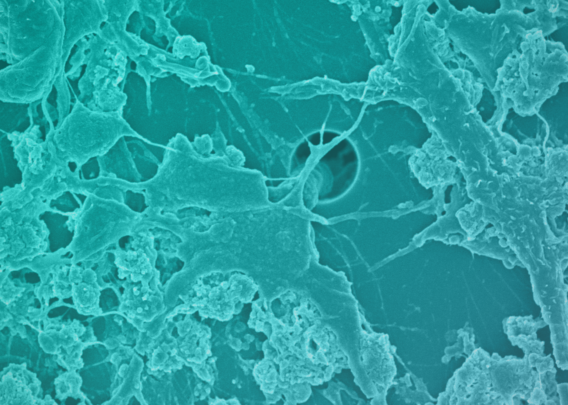
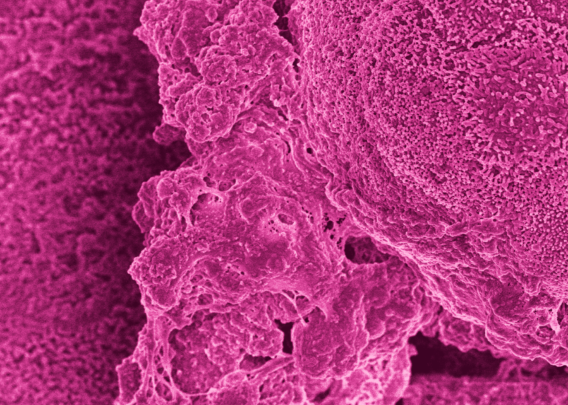
This advanced, in vitro intestine model incorporates primary human biopsy tissue cultured into organoids. Critically, the cells retain their “stemness,” meaning they replicate the stem cell niches that are constantly regenerating in human intestines.
After the organoids are dissociated, they are seeded in the top channel of the Organ-Chip. The bottom channel contains primary human intestine-derived microvascular endothelial cells, which are in close proximity to the epithelial cells, as they would be in vivo. The channels are separated by a porous membrane coated with tissue-relevant extracellular matrix proteins.
From there, mechanical forces on the chip—physiologic flow and cyclic stretch—replicate intestinal peristalsis, which improves cell morphology and functionality while supporting more accurate gene expression.
As a result, epithelial tissues respond to microvasculature cues, and the epithelial cells differentiate into all three major epithelial types at the appropriate ratios.
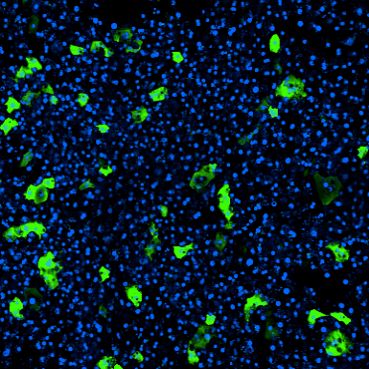
With this, the Emulate Colon Intestine-Chip is able to model IBD from the initial insult to the cytokine cascade, demonstrating along the way selective immune cell recruitment, cell death, and tight junction loss. This model can be applied to study inflammation-specific immune recruitment from vasculature into epithelial tissue and subsequent downstream impacts.
“We have shown that this Organ-Chip strongly reflects what we see in primary human tissue,” said Carman. “It develops proper tight junctions and a strong functional barrier. On the molecular level, we see transcriptional signatures that are highly reflective of primary human tissue.”
This model has demonstrated the efficacy of small molecule inhibitors that target IFNγ and IL-22 signaling pathways, meaning researchers can use it to validate clinically relevant drug candidates designed to prevent barrier dysfunction.
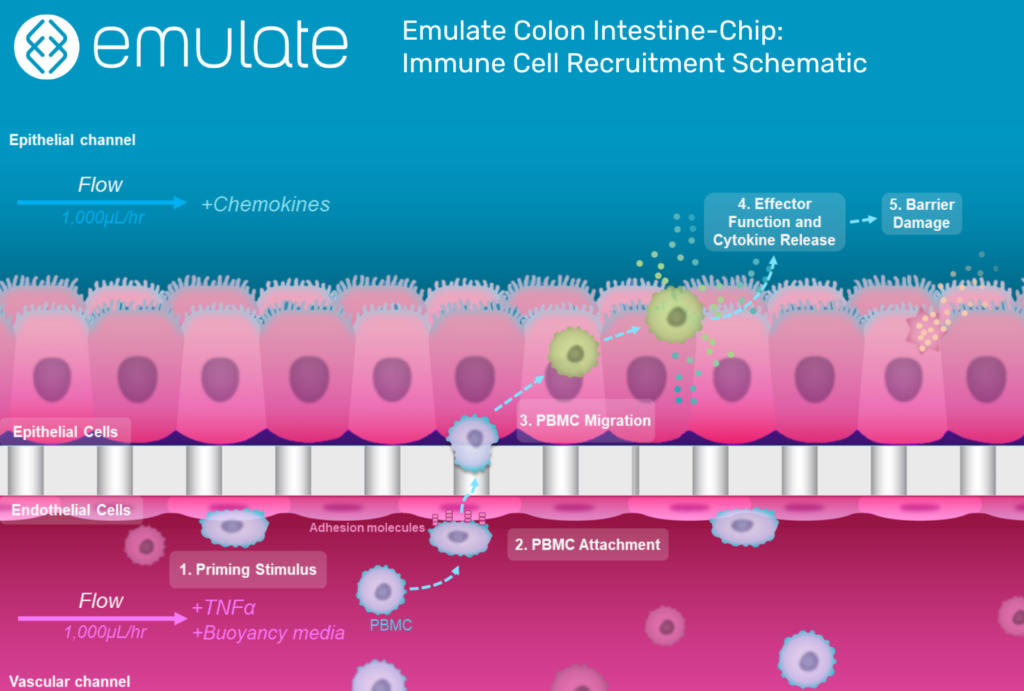
Selectively Generating Inflammation
One of the Organ-Chip’s most important abilities is the selective recruitment of immune cells. This selectivity comes from tissue-specific adhesion molecules on both endothelial and immune cells, which must be highly specific to bind.
Around 30% of the body’s circulating immune cells are customized for work in the intestines. They have a molecule called α4β7 integrin that binds to an endothelial molecule called MAdCAM-1, which is preferentially expressed in the colon endothelium and upregulated in response to inflammatory cues.
One of the major ways the Colon Intestine-Chip replicates IBD biology is by expressing MAdCAM-1 in response to inflammatory stimuli, giving it tremendous relevance for therapeutic discovery.
“The α4β7 integrin/MAdCAM-1 adhesion molecule axis is an important therapeutic target,” said Carman. “If we can interfere with that adhesion, we can potentially interrupt the inflammatory cascade. And because this mechanism is selective to the gut, any therapeutic that targets these adhesion molecules would be highly specific to the intestinal system.”

“One drug, AJM300, is in phase three clinical trials right now and is showing promising safety and efficacy,” said Carman. “We validated that efficacy in our model. We also used the model to study the corticosteroid dexamethasone, which has been a mainstay in IBD treatment for many years. We recently published the data in an application note.”
The Colon Intestine-Chip provides a more complete picture of human IBD pathogenesis, delivering a human-relevant platform to test drug efficacy. However, for Emulate, it’s just the beginning. Inflammation plays a major role in many conditions, and creating models that effectively replicate those pathways will be essential in validating and advancing therapeutic compounds to support better care.
“This IBD model is our first foray into inflammation,” said Carman. “We’re planning on developing many variations on this theme to create better tools for a variety of inflammation-driven indications.”
For more information on Emulate’s IBD model, please download Modeling Inflammation-Specific Immune Cell Recruitment in the Colon Intestine-Chip.