Organ-Chip Application
Organ-Chips for Toxicology Assessment
Confidently predict the human toxicity of drug candidates with Organ-Chips
YOUR CHALLENGE
Accurately predict the toxicity of preclinical drug candidates
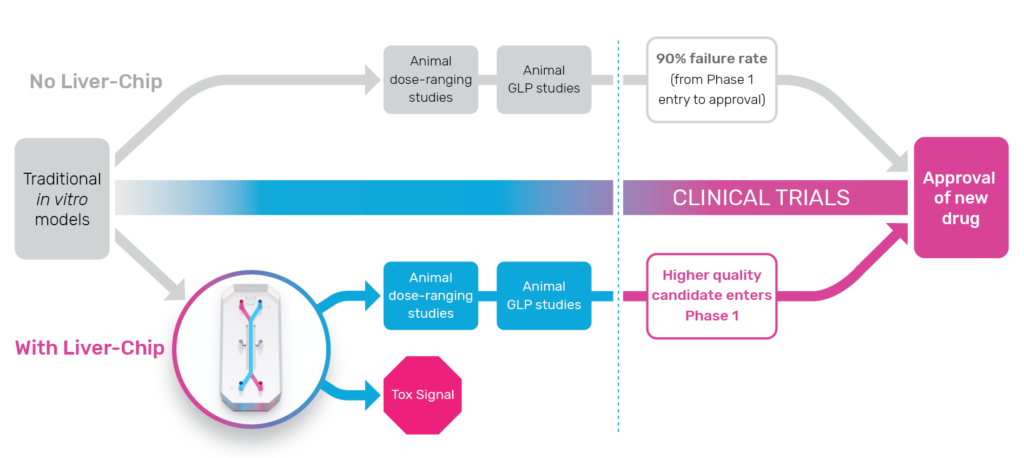
Biopharmaceutical companies encounter many challenges in developing human drugs, but evaluating whether they’ll be toxic in the clinical stage can be particularly challenging. Approximately 30% of drugs fail during human trials due to toxicity—despite having passed preclinical safety screenings in animals. Put simply, conventional models lack the predictive value required to confidently transition drug candidates to the clinic.
How we can help
Translate to the clinic with confidence by predicting human response earlier
The predictive power of Organ-on-a-Chip technology enables researchers to accurately evaluate the human-relevant profile of preclinical drug candidates and effectively model clinical outcomes. Earlier prediction of human response can provide safer drug candidates, reduce the number of animals needed for testing, and generate billions of dollars per year due to increased R&D productivity.
HEPATOTOXICITY
The first human Liver-Chip qualified against IQ MPS guidelines for predicting drug-induced liver injury
Drug-induced liver injury (DILI) is one of the primary toxicities that cause drugs to be withdrawn from the market and remains a 21st century patient safety concern.
To measure how much Organ-Chips could improve patient safety, researchers qualified the Emulate human Liver-Chip against the guidelines defined by IQ MPS, an affiliate of the International Consortium for Innovation and Quality in Pharmaceutical Development. The study demonstrated that the Emulate human Liver-Chip was able to correctly identify 87% of the tested drugs that caused drug-induced liver injury in patients despite passing animal testing evaluations. At the same time, the Emulate human Liver-Chip did not falsely identify any drugs as toxic leading to a 100% specificity and supporting its use in toxicology screening workflows.
Implementing the Emulate human Liver-Chip in Preclinical Workflows
Improving the lead optimization phase
Researchers can use the Emulate human Liver-Chip in the lead optimization phase of their drug development pipeline, where projects typically identify three to five chemical compounds that have the potential to become a candidate drug. By doing so, a chemical compound that produced a toxic signal in the Emulate human Liver-Chip could be deprioritized from early in vivo studies, reducing animal testing and permitting safer candidates to progress through the development pipeline.
To demonstrate how using Organ-Chips in preclinical workflows can improve outcomes in clinical trials, consider this use case: TAK-875 was a drug candidate discontinued during phase III trials due to DILI. When the compound was retrospectively studied on the Emulate human Liver-Chip, results showed that prolonged exposure to the compound caused mitochondrial dysfunction, oxidative stress, lipid droplet formation, and an innate immune response—all harbingers of DILI for susceptible patients. Had the Emulate human Liver-Chip study been performed prior to clinical trials, researchers could have found human-specific toxicity concerns earlier, deprioritized TAK-875 as a drug candidate, and moved forward with safer candidates.
renal toxicity
Predicting Drug-Induced Nephrotoxicity
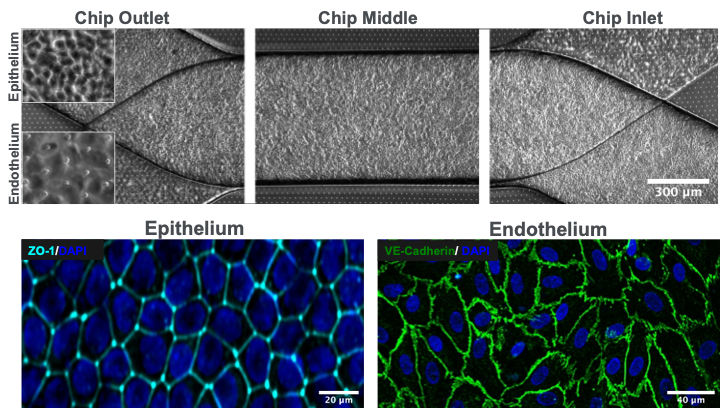
Drug-induced nephrotoxicity accounts for a majority of acute kidney injury (AKI) cases and is a primary cause of clinical attrition for lead therapeutic candidates. The lack of clinically predictive tools that are sensitive enough to detect early biological markers of kidney injury is a key factor in the failure to translate preclinical results into successful clinical outcomes. The Emulate human Kidney-Chip solves this challenge by recreating key physiological features of the human kidney, such as the tubular-peritubular interface, to more accurately model in vivo physiology. This model expresses transporters that are key to proper kidney function in vivo, and it demonstrates appropriate toxic responses to well-known nephrotoxicants at physiologically relevant concentrations.
Intestinal toxicity
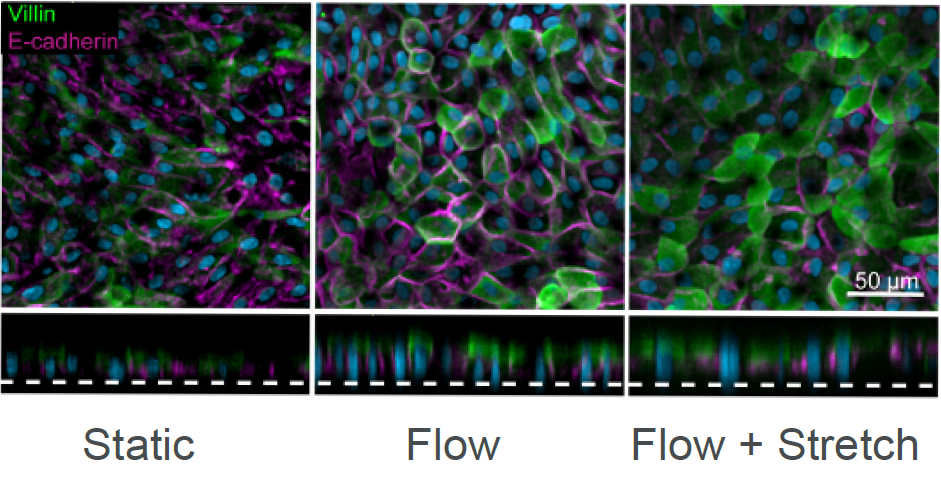
Assessing drug safety using a human Duodenum Intestine-Chip
Current in vitro preclinical models, including Caco-2 2D models and organoids, often fail to accurately determine intestinal toxicity, presenting an unmet need for better models in order to improve clinical success. The Emulate human Duodenum Intestine-Chip recreates intestinal tissue and in vivo-like physiological behaviors to assess drug safety. A range of experiments and assays can easily be accomplished with the Emulate human Duodenum Intestine-Chip for real-time and post-study analysis, including LDH assays, effluent testing, and transcriptomic analysis.
our offering
A range of Organ-Chip models to study drug toxicity profiles in a human-relevant system
During preclinical drug development, Organ-on-a-Chip technology can be used to study the toxicity profiles of drug candidates in validated models of the liver, kidney, and intestine.
Benefits
Screen for diverse mechanisms of toxicity for preclinical drug candidates
Assess the human relevance of toxicity observed in preclinical animal studies
Accurately model dose-response curves by treating at clinically relevant concentrations
Identify the mechanism(s) of toxicity for candidates with unexpected safety signals in the clinic
Supported Organ Models
Liver-Chip
Investigate mechanisms of hepatotoxicity for preclinical drug candidates to help decrease clinical trial safety failures due to DILI.
Kidney-Chip
Evaluate the nephrotoxicity of drug candidates at clinically relevant concentrations in a co-culture human kidney model.
Duodenum Intestine-Chip
Emulate human intestinal tissue and recreate in vivo-like physiology for use in the assessment of drug safety.
FEATURED RESOURCE
Liver Toxicology White Paper
This white paper outlines the benefits of incorporating the Liver-Chip S1 into preclinical workflows and its potential to reduce costs and bring safer drugs to market faster. It also introduces the Liver-Chip R1, a new liver model designed to improve testing accuracy for lipophilic drugs by reducing drug absorption, further enhancing predictive toxicology study precision.






