In the last decade, the realm of cancer therapy has taken groundbreaking strides, with Chimeric Antigen Receptor (CAR) T-cell therapy being one of the most promising new types of treatment. Having shown potential in treating blood cancers, CAR T-cell therapies are now setting their sights on a more complex adversary: solid tumors. This article explains what CAR T-cell therapy is, why solid tumors are such a challenging target, and how researchers can use advanced in vitro models to gain a more human-relevant understanding of CAR T efficacy.
Understanding CAR T-Cell Therapy
At its core, autologous CAR T-cell therapy is a form of immunotherapy where a patient’s own immune cells are modified so they recognize and kill cancer cells more effectively. This is done by adding a gene for a receptor, called a chimeric antigen receptor (CAR), which is designed to identify a specific tumor antigen. These modified cells are infused back into the patient, where they hunt down and kill the cancer cells.
To date, researchers have successfully developed six FDA-approved CAR T-cell therapies for blood cancers, including leukemia, lymphoma, and multiple myeloma. This has proved invaluable for those in difficult-to-treat patient populations who exhibit little to no response to conventional therapies.
However, these hematological malignancies only represent a small portion of all cancer cases and deaths—about 90% of cancers are solid tumors. Unfortunately, efforts to adapt CAR T-cell therapy for solid tumors have, to date, proved challenging.
Stay Up to Date with the Emulate newsletter
The Challenges of CAR T for Solid Tumors
High-mortality, treatment-resistant solid tumors—including lung, breast, colorectal, and pancreatic cancer—are prime candidates for immunotherapy. However, the relative complexity of the heterogeneous solid tumor environment creates novel immunological obstacles that are not seen in blood cancers.
The challenges are many, but a few highlights include:
- Selective Trafficking and Migration: For blood cancers, immunotherapies are administered in the same location where the cancerous cells reside—the circulatory system. But for solid tumors, this is just the start of the journey. After being administered into the blood stream, the immune cells must selectively traffic out of the vasculature at the site of the tumor and then migrate to the tumor tissue. This is a challenging but critical journey, as the CAR T cells must reach the tumor in adequate numbers to effectively kill the cancerous cells, while avoid attacking healthy tissue.
- Tumor antigen heterogeneity: In hematological tumors, antigen expression is relatively stable and uniform. But in sharp contrast, solid tumors can exhibit significant variability in antigen expression, both within a single tumor and between patients. This means that CAR T-cell therapy targeting a specific antigen might only attack certain parts of the tumor, leaving other parts untouched. It may also present the need for a more personalized or multi-antigen-targeted CAR T approach.
- Tumor Microenvironment (TME): The area surrounding solid tumors is a unique cellular and chemical landscape, where inhibitory immune cells, immune checkpoints, and cytokines create an environment that is naturally hostile to immune cells, including CAR T-cell therapy. This means that once CAR T cells reach the tumor, they can quickly become suppressed or inactivated by immunosuppressive factors in the TME, hindering their ability to effectively destroy cancer cells.
The Need for Human-Relevant Research Models
While enthusiasm for solid tumor CAR T-cell therapy is strong, FDA-approved therapies have so far remained out of reach largely due to the challenges described above. A major limiting factor in overcoming these challenges has been a lack of preclinical research models that can adequately recreate the complex journey that CAR T cells must undertake in the human body to be effective. The models that researchers rely on for developing immunotherapy fall into two main categories: in vitro models and animal models.
2D cell models often include tumor cell lines or patient-derived organoids (PDO) cultured in a static dish, where the CAR T cells are be administered directly to the tumor cells to evaluate killing efficacy. However, these models only allow researchers to evaluate the end of the CAR T cell journey and completely ignore the aspect of CAR T cell tracking and migration.
Animal models remain the cornerstone of preclinical immunotherapy testing and have provided important contributions to hematological cancer CAR T-cell therapies that are on the market today. One example is patient-derived xenografts (PDX) models, in which human patient-derived tumors are implanted in immunodeficient mice. However, while animal models enable a more complete understanding of immunotherapy effect—allowing the CAR T cells to be intravenously administered and traffic to the tumor as they would in humans—they suffer from large species differences in responses and relatively poor insights into mechanism of action, limiting their successful translation to human clinical response.
To truly understand the dynamics of cancer immunotherapy for solid tumors and develop effective treatments, researchers need more advanced models that can capture the complex intricacies of human biology and immune response.
A Path Forward for Solid Tumor Immunotherapy Development
For CAR T-cell therapy’s potential to be fully realized in solid tumors, it is vital that its therapeutic candidates’ behavior, efficacy, and challenges be studied in a context that is as close to human as possible.
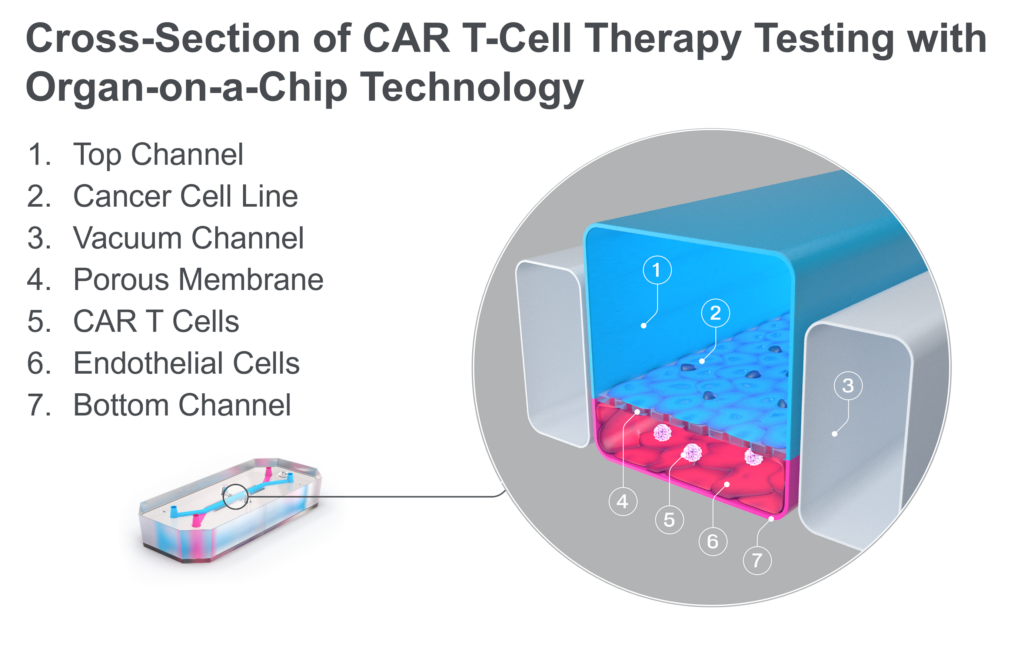
Fortunately, Organ-on-a-Chip technology can offer a path forward. Organ-Chips are microengineered devices that emulate the architecture, functionality, and physiological responses of vascularized human organs. They provide a dynamic platform that closely replicates human tissue-tissue interfaces, fluid flow, and mechanical forces, creating an environment where CAR T cells can be studied in a setting that more accurately mirrors the human body.
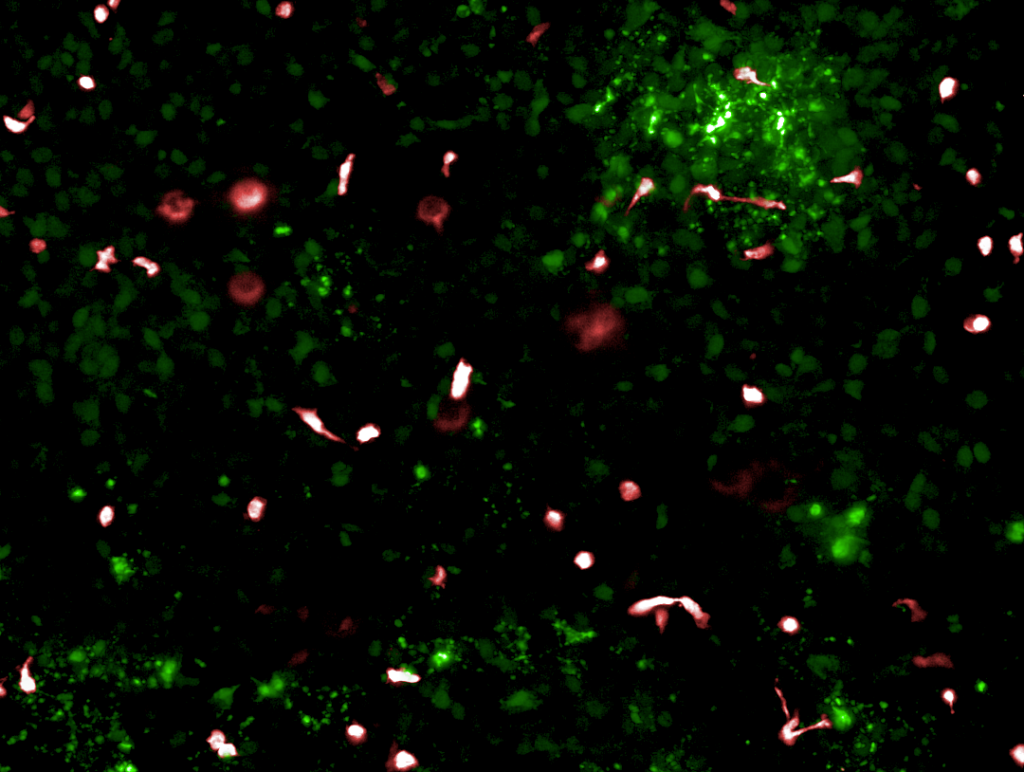
Recently, this technology has been used to study the efficacy of CAR T-cell therapy in a model of non-small cell lung carcinoma (NSCLC). By co-culturing an NSCLC cell line and lung-specific vascular cells, researchers were able to model the journey that CAR T cells undergo in vivo. After administering the immunotherapy in the chip vasculature—just like its intravenous administration in the body—researchers saw how the CAR T cells attached to the vasculature, migrated to the tumor cell-containing channel, and subsequently exhibited antigen-dependent killing of cancer cells. Through imaging and effluent analysis, they could even quantify the amount of migration and killing as well as the levels of exhaustion markers for CAR T cells. A common treatment approach of administering a co-therapeutic alongside CAR T cells was even modeled on-chip, where it demonstrated improved CAR T-cell migration to the site of the solid tumor cell line. As this technology becomes more widespread, it may enable immunotherapy companies to accelerate the successful development of the first solid tumor CAR T-cell therapy.
Conclusion
Solid tumors continue to present numerous challenges for CAR T-cell therapy researchers, including efficient CAR T cell trafficking, migration, and infiltration, as well as overcoming the hostile tumor microenvironment.
As researchers look to surmount these obstacles, advanced cell culture technologies like Organ-Chips may provide a more human-relevant and predictive assessment of CAR T cell efficacy, improving translation from pre-clinical testing to clinical success. With a number of CAR T-cell therapies in development around the world, the horizon seems promising, providing hope that researchers may be able to widen the reach of CAR T cells beyond blood cancer and into the more formidable realm of solid tumors.