The hallmarks of progressive neurodegenerative diseases—problems with mobility and coordination or lapses in memory—are sadly familiar to millions of people. As neurons and supporting cells of the central and peripheral nervous system die, symptoms progressively get worse and are ultimately fatal for many of those who suffer from conditions like Alzheimer’s, Parkinson’s, or amyotrophic lateral sclerosis (ALS). Although the precise genetic, cellular, and physiological causes of all progressive neurodegeneration are not fully understood, misregulated inflammation has been implicated in many of them.
The global healthcare burden of neurodegenerative diseases remains extraordinarily high. According to the American Journal of Managed Care (AJMC), in 2020 nearly 6 million Americans suffered from Alzheimer’s disease with the estimated cost of care at $305 billion. This cost is expected to increase to more than $1 trillion per year as the population ages and disease prevalence grows. Although biopharmaceutical companies are urgently working to develop successful therapies and treatments, clinical trial failure rates remain high — Alzheimer’s in particular has a 100% failure rate for disease-modifying therapies. The human brain and nervous system are notoriously complex, and many gaps in understanding the mechanisms of disease progression, drug action, toxicity, and pharmacokinetic profiles continue to plague preclinical research reliant on conventional in vitro cell culture and animal models. Despite vast amounts of resources invested in drug development, companies are struggling to streamline the process and bring safer, more effective treatments for neurodegenerative disease to market faster.
Technology Development: Animal and In Vitro Models for Neuroscience Research
The use of animal models for neuroscience research has a long history, dating back to the work of Aristotle. Looking at animal brains as proxies for human brains has yielded many important discoveries, including characterizing neural cell types and how they contribute to organ structure and function, defining developmental patterns, and studying the effects of aging and drugs. While there are many good animal models for examining toxicity, drug effects, or motor and sensory functions, distinct species differences between animals and humans have hindered successful translation of neurogenerative therapies to the clinic.
Over the past two decades, advances in in vitro cell culture have fueled a hopeful new era in neurodegenerative disease research. Co-culture models that incorporate endothelial cells, astrocytes, and pericytes to mimic the blood-brain barrier (BBB) have been developed to examine uptake of drug candidates or examine potential toxicity or cellular injury. These two-dimensional systems are useful for simple experiments that have defined endpoints, but the cultures typically lack physiological complexity in cell type, structure, and dynamic forces, limiting their predictive value. Breakthroughs in induced pluripotent stem cell (iPSC) research in the mid-2000s paved the way for culturing and characterizing many specialized human neural cell types for the first time. Alongside these developments, three-dimensional models called organoids, derived from stem cells, emerged with features like advanced cell composition, differentiation, and tissue architecture. Brain organoids have tremendous potential for understanding the developmental patterns of cells and how they are affected by genetic and environmental factors implicated in disease. However, even the most advanced co-cultures and organoids fall short in modeling the neurovascular unit as they lack a functional vascular system and usually, the relevant cell types involved in signaling, transport, and immune recruitment.
Despite these advances in in vitro culture, more predictive models that reduce translational failures from preclinical testing to the clinic are needed to speed therapeutic development for neurodegenerative conditions like Alzheimer’s.
A More Predictive In Vitro Model of the Human Brain
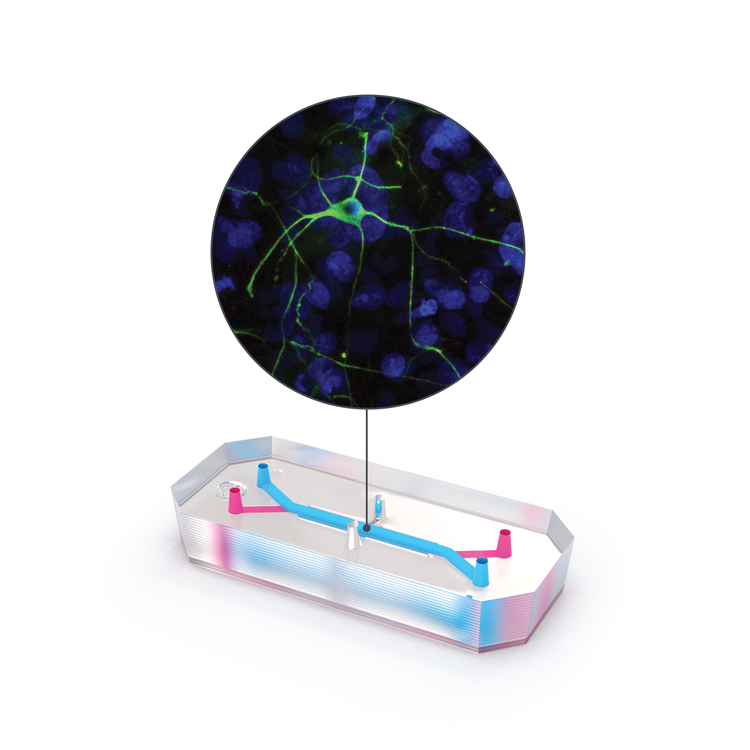
For researchers investigating neuroinflammatory disease and therapeutics, Organs-on-Chips technology can provide a better model of the human brain and help elucidate mechanisms of disease, the inner workings of the blood-brain barrier, drug toxicity, and drug efficacy. The Emulate Brain-Chip is under development as a comprehensive model of the neurovascular unit that incorporates cells from the human brain—including the BBB—in a dynamic microenvironment. This “Brain-on-a-Chip” features five human iPSC-derived and primary cell types (neurons, microglia, astrocytes, pericytes, and brain microvascular endothelial cells) in a dynamic, tunable microenvironment. This complex human model can support the co-culture and establishment of extensive interaction between human iPSC-derived neurons and primary glial cells (astrocytes and microglia) and reproduce key features of neuroinflammation after exposure to inflammatory stimuli. Human iPSC-derived brain endothelial cells are successfully maintained in the vascular channel of the Brain-Chip in the presence of fluidic shear stress, while exhibiting hallmark features of the human blood-brain barrier, such as development of tight junctions and minimal barrier permeability. Taken together, the co-culture of these cell types in a dynamic microenvironment results in improved functionality, stability, and in vivo-like gene expression.
The Emulate Brain-Chip is the next step in predictive biological models for neurodegenerative disease research, enabling researchers to investigate the safety, efficacy, and BBB penetration of therapeutics in a comprehensive, human-relevant model. Clinical successes enabled by the Brain-Chip will bring much-needed hope to millions who are impacted by neurodegenerative diseases.
Contact us today to learn more about how Organ-on-a-Chip technology can help you advance your neurodegenerative disease research.