In this blog, we’re recapping the exciting highlights from the 4th annual MPS World Summit conference that took place in Brussels, Belgium from June 9-13. Read on to catch up on the latest technology and innovative research that was unveiled at the conference!
Global Launch of the AVA Emulation System: The Next Evolution of the Human-Centric Era
The 2025 MPS World Summit marked the official introduction of the AVA Emulation System, a next-generation 3-in-1 Organ-Chip platform designed specifically for high-throughput Organ-Chip experiments. By combining microfluidic control for 96 Organ-Chip “Emulations” with automated imaging and a self-contained incubator, AVA unlocks the scale needed for pharma and translational researchers to move from pilot studies to robust, reproducible data generation.

Live demos at the Emulate booth drew consistent crowds, as researchers saw firsthand how AVA enables:
Expanded experimental power – 96 independent Organ-Chip samples in a single run enable researchers to achieve microplate-level scale of their Organ-Chip experiments, enabling side-by-side comparison of dozens of compounds, doses, or stimuli.
Lower operating costs – AVA achieves a four-fold drop in consumable spend and up to 50% fewer cells and media per sample compared to previous generation technology.
Time & labor savings – Hands-on, in-lab time is reduced by more than half, thanks to automated microscopy, remote monitoring, and the revolutionary new Chip-Array™ consumable that integrates 12 independent Organ-Chips into an SBS format for streamlined workflows with multichannel pipettes and automated liquid handlers.
AI-ready datasets – A typical 7-day experiment can generate >30,000 time-stamped data points from daily imaging and effluent assays, with post-takedown omics pushing the total into the millions—providing a rich, multi-modal foundation to feed machine-learning pipelines for target discovery, lead optimization, and safety prediction.


The response was overwhelmingly enthusiastic! Both academic and industry users expressed excitement about increasing throughput, reducing manual workflows, and finally having a robust platform that gives users the practical means to pivot beyond animal testing and into truly human-relevant territory. Several pharma partners noted AVA’s potential to help bridge the gap between exploratory MPS modeling and routine, high-throughput testing.
Chip-R1 Makes its MPS World Summit Debut
Last September, Emulate launched a new consumable for the Zoë-CM2 Culture Module, the Chip-R1 Rigid Chip. This non-PDMS consumable is constructed with minimally drug-absorbing plastics, making it well suited for ADME and toxicology applications. In addition to its low-drug-absorbing properties, Chip-R1 also features a shorter vascular channel. This modified design enables physiologically relevant levels of shear stress to be applied, which is critical for applications such as immune cell recruitment.
At MPS2025, Emulate’s Director of Discovery Biology Randy Daughters presented a poster on the development of Chip-R1, and how it improves precision for toxicology and ADME studies. Check out the poster here!
A Convergence of Academia, Government, and Industry: Emulate Organ-on-a-Chip Technology in Action
In addition to introducing AVA to the world, MPS2025 showcased an extraordinary array of scientific advancements using Emulate’s Organ-on-a-Chip technology. From academia to industry and government labs, researchers unveiled how microphysiological systems (MPS) are reshaping the way we model disease, predict drug responses, and accelerate discovery.
This year, more than 15 posters and multiple talks featured models based on Emulate’s Chip S1® Stretchable Chip and Chip A1™ Accessible Chip, covering diverse organ systems and applications. Here’s a look at the emerging research themes and standout case studies:
1. Pushing Preclinical Boundaries in Pharma
Inflammatory Bowel Disease (IBD) Models
- AbbVie used an Intestine-Chip to study the impact of therapeutic intervention on goblet cells and barrier integrity in IBD.
- Institut Pasteur presented a new intestinal inflammation-on-chip model aimed at identifying novel IBD therapies.
- London South Bank University explored hydrogel-based IBD cell therapy efficacy in an Intestine-Chip model.
Liver and Kidney Safety Assessment
- Boehringer Ingelheim and Daiichi Sankyo advanced the use of Liver-Chip systems for cross-species DILI prediction and comparative liver toxicity.
- UCB validated a Kidney-Chip model for antisense oligonucleotide de-risking, a rising modality in pharma pipelines.
Lung Safety and Toxicity
- Boehringer Ingelheim also qualified a human Alveolus Lung-Chip for antibody drug conjugate (ADC) safety in patients with risk factors—pointing to the importance of patient-derived chips in regulatory science.
Immunosafety Innovation
Pfizer shared data on a Lymph Node-Chip capable of predicting antigen-specific immune responses—a major leap for preclinical immunotoxicity testing.
2. Blood-Brain Barrier Modeling & Neurological Safety
Bayer developed a BBB-Chip for translational studies, aiming to bridge the gap between in vitro prediction and in vivo outcomes—especially critical for CNS drug development.
AFRL (U.S. Air Force Research Laboratory) used Brain-Chip platforms with machine learning to rapidly detect neurotoxin exposure and evaluate interventions—highlighting chip utility in national defense and safety applications.
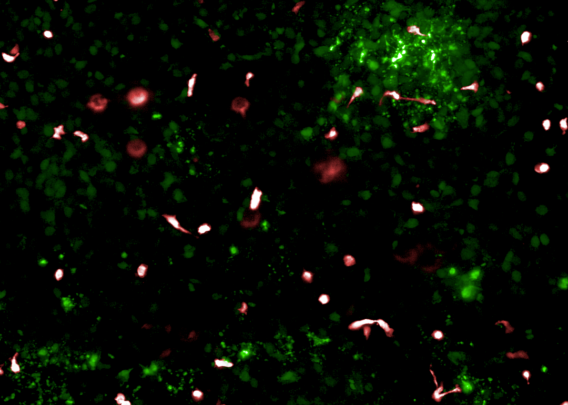
3. Recreating Infectious Disease Dynamics in the Lung
Lung Infection-on-a-Chip
Institut Pasteur unveiled a comprehensive infection model using lung-derived airway and alveolar organoids cultured on Chip-S1. Their findings:
- Demonstrated infection and barrier disruption from Streptococcus pneumoniae strains.
- Revealed the replication fitness of SARS-CoV-2 variants, with Delta infecting alveolar type II cells while Omicron BA.5 failed to replicate effectively.
- Highlighted strong innate immune responses, even from low-replicating strains—underscoring the chip’s ability to capture subtle immune dynamics.
View the full poster here!
UK Health & Safety Agency also modeled pandemic pathogens in alveolar MPS at high containment, testing real-world countermeasures in a controlled chip format.
4. Modeling Disease Complexity Across Human Tissues
Musculoskeletal Innovation
Queen Mary University of London led the charge with five posters spanning osteoarthritis, tendon repair, and bone metastasis. They presented:
- A personalized synovium-cartilage chip for understanding patient-specific inflammation in osteoarthritis.
- A tendon-on-a-chip that leverages physical cues to mimic musculoskeletal architecture.
- A bone metastasis model integrated with multi-omics tools for tracking osteolytic changes.
Bone Marrow-on-a-Chip
Azmeer Sharipol of the University of Rochester Medical Center shared a cutting-edge bone marrow chip used to study acute myeloid leukemia in vitro—pushing forward personalized oncology research.
5. Tools & Materials Innovation
Several teams (QMUL, Pasteur, and others) showcased innovations in hydrogel engineering, growth factor patterning, and custom matrix development to better control cell behavior and tissue architecture on-chip—further strengthening the in vivo-relevance of these MPS systems.
The 2025 MPS World Summit made one thing clear: Organ-on-a-Chip is no longer an emerging tool—it’s an established, versatile platform for real-world biomedical problems. Whether in drug safety, infectious disease, personalized medicine, or systems biology, Emulate’s chips are helping scientists go beyond conventional models to unlock new possibilities in human-relevant research.
Ready to explore what Organ-on-a-Chip can do for your research?
Learn more about Emulate’s portfolio and how our platforms are accelerating discovery across therapeutic areas by downloading our brochure.