Drug candidates are usually discovered or developed with a specific purpose in mind: to stop infections, to target and kill cancer cells, or to repair a faulty cellular pathway. Sometimes, once these approved treatments are taken by large groups of people, clinicians may observe unexpected and exciting benefits. For example, the drug Raloxifene was developed to prevent and treat osteoporosis by mimicking the effects of estrogen to increase bone density. Through retrospective clinical analysis, the drug was also shown to decrease the risk of developing invasive breast cancer by blocking estrogen’s effect on breast tissue.
Drugs that inhibit viral infection have been successfully developed and in use for decades, and with the current SARS-CoV-2 crisis, speeding up testing of potential preventive therapies is an urgent priority. One of the fastest ways to respond to a global challenge like a pandemic is to repurpose existing, approved drugs as treatments for emerging pathogens.
Organ-Chips Identify Potential Prophylactic Treatment for COVID-19
During the past year, many research groups around the world have focused on drug repurposing for COVID-19 using established cell lines, primary tissue-derived human cells, organoids, and explanted human lung tissue cultures. However, it is well known that cells grown in a two-dimensional dish do not behave like the cells in a living human body, and many drugs that appear effective in pre-clinical lab studies do not work well in patients.
In a collaborative effort to test whether existing drug candidates block infection from respiratory pathogens like SARS-CoV-2, researchers from the Wyss Institute for Biologically Inspired Engineering at Harvard University, the University of Maryland School of Medicine, the Icahn School of Medicine at Mount Sinai and others used Emulate technology to develop an Airway Lung-Chip to examine eight existing candidate therapeutics for SARS-CoV-2, including hydroxychloroquine and chloroquine, that they and others had previously observed in conventional cell culture assays. The study is reported in Nature Biomedical Engineering.
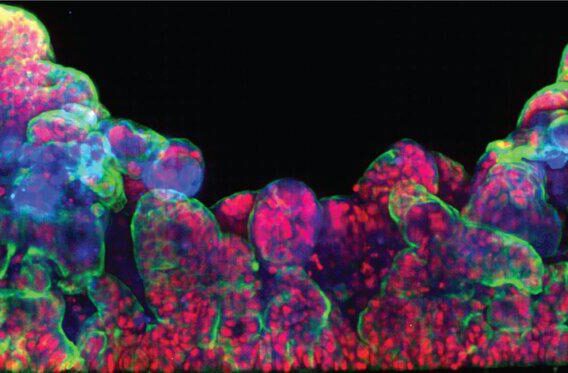
The Emulate Airway Lung-Chip used for these studies is a small microfluidic culture system device with two parallel channels separated by a porous membrane. Human lung airway cells are grown in one channel exposed to air, while human blood vessel cells are grown in the other channel exposed to cell culture medium to mimic blood flow. Cells in the Airway Lung-Chip differentiate into specific types similar to those in the human airway and develop lung-specific traits such as cilia and the ability to produce and move mucus. The cells also express angiotensin-converting enzyme-2 (ACE2) receptors on their surface, which is co-opted by the virus to gain entry into cells.
When the cells in the Airway Lung-Chip were infected with a non-infectious SARS-CoV-2 virus, most of these drugs, including hydroxychloroquine and chloroquine, were not effective at stopping infection. However, one antimalarial drug called amodiaquine reduced infection by 60 percent by preventing viral entry.
Subsequent Research Suggests Drug’s Potential Therapeutic Effects
Despite the promise of amodiaquine, the team still needed to demonstrate that it worked against the infectious SARS-CoV-2 virus. Virologists Matthew Frieman, PhD at the University of Maryland School of Medicine and Benjamin tenOever, PhD at the Icahn School of Medicine at Mount Sinai, had both the expertise and laboratories designed to study infectious pathogens and were eager to collaborate with the Wyss Institute. The Frieman lab tested amodiaquine and its active metabolite, desethylamodiaquine, against native SARS-CoV-2 via high-throughput assays in cells in vitro and confirmed that the drug inhibited viral infection.
In parallel, the tenOever lab tested amodiaquine and hydroxychloroquine against native SARS-CoV-2 in a head-to-head comparison in a small animal COVID-19 model and saw that prophylactic treatment with amodiaquine resulted in an approximately 70 percent reduction in viral load upon exposure, while hydroxychloroquine was ineffective. They also saw that amodiaquine prevented the transmission of the virus more than 90 percent of the time, and that it was also effective in reducing viral load when administered after introduction of the virus. Thus, their results suggest that amodiaquine could work in both treatment and prevention modes.
“Seeing how beautifully amodiaquine inhibited infection in the Airway Lung-Chip was extremely exciting,” said Frieman. “The fact that it seems to work both before and after exposure to SARS-CoV-2 means that it could potentially be effective in a wide variety of settings.”
“This collaboration has allowed us to do things that we never would have had the resources to do otherwise, including recently setting up Organ-Chips in our own lab so that we can now use them to study the interactions between infectious viruses and their hosts. While we’re proud of what we’ve accomplished so far for COVID-19, we’re also looking forward to studying additional virus-host dynamics using the Organ-Chips in the hopes that we’ll be able to prevent or address future pandemics,” said tenOever, who is a Professor of Microbiology.
In Vivo Testing Continues
The Organ-Chip-based testing ecosystem greatly streamlines the process of evaluating the safety and efficacy of existing drugs for new medical applications and provides a proof-of-concept for the use of Organ-Chips to rapidly repurpose existing drugs for new medical applications, including future pandemics. Amodiaquine is now in clinical trials for COVID-19 at multiple sites in Africa, where this drug is inexpensive and widely available.
“The collaboration with the Wyss Institute and the Frieman and tenOever labs has created a preclinical drug development pipeline that produced clinically significant results with incredible speed,” said Lorna Ewart, EVP of Science at Emulate. “With customizable Organ-Chip technology in the hands of researchers, preclinical testing of new drug candidates or repurposed drugs can be further accelerated with more human-relevant models, allowing us to be better prepared to confront this and future pandemics.”
For more information on how Emulate products are being used to study COVID-19, read our blog post titled How Organs-on-Chips Technology is being used to Provide Scientific Insights into COVID-19.