Organ-Chip Application
Neuroscience Research & Drug Development
Advance drug discovery and development for neurodegenerative diseases
YOUR CHALLENGE
There is an unmet need for effective neurodegenerative disease therapeutics
The human brain and central nervous system are notoriously complex. To date, preclinical research has been limited by conventional in vitro cell culture and animal models, limiting our understanding of disease progression, drug efficacy, toxicity, and pharmacokinetic profiles. This has resulted in lack of effective disease-modifying therapies for many brain diseases, such as Alzheimer’s. To accelerate the development of safe, more effective treatments for neurodegenerative disease, a more physiologically relevant model of the human brain is needed.
How we can Help
Unravel the complexity of the neurovascular unit
Organ-on-a-Chip technology can provide a more comprehensive model of the human brain neurovascular unit, enabling a more accurate understanding of neurodegenerative disease mechanisms, elucidating the inner workings of the blood-brain barrier, and increasing the translation of preclinical drug studies to human response.

Blood-Brain Barrier Penetration
Improve therapeutic delivery to the brain with a human-relevant in vitro neurovascular unit barrier model
A major challenge in neuroscience is designing drugs that can pass through the blood-brain barrier (BBB). While animal models may be suitable for passive transport studies, species differences in BBB transporter and receptor expression often limit the human relevance of active transport studies. Meanwhile, conventional in vitro BBB models typically lack adequate cellular complexity, resulting in differences in permeability and transporter expression that limit clinical translation. The Brain-Chip is under development to overcome these obstacles by incorporating five human cell types in a single model—including brain microvascular endothelial-like cells—resulting in low permeability and high gene expression of transporter pathways.
NEUROINFLAMMATION
The most comprehensive in vitro model of the human neurovascular unit to study mechanisms of neuroinflammation and investigate the safety and efficacy of therapeutics
Though animal models have yielded many insights into human neurophysiology, species differences have limited successful translation of neurodegenerative therapies to the clinic. Even advanced in vitro models such as brain organoids typically lack microglia, media flow, and vasculature, critical for studying disease impact on the blood-brain barrier. The Brain-Chip incorporates microglia, essential for modeling neuroinflammation, and also contains both the neuronal compartment and brain microvascular endothelial-like cells, so researchers can study the effect of neuroinflammation and drug candidates across the entire neurovascular unit.
How Organ-Chips are being used
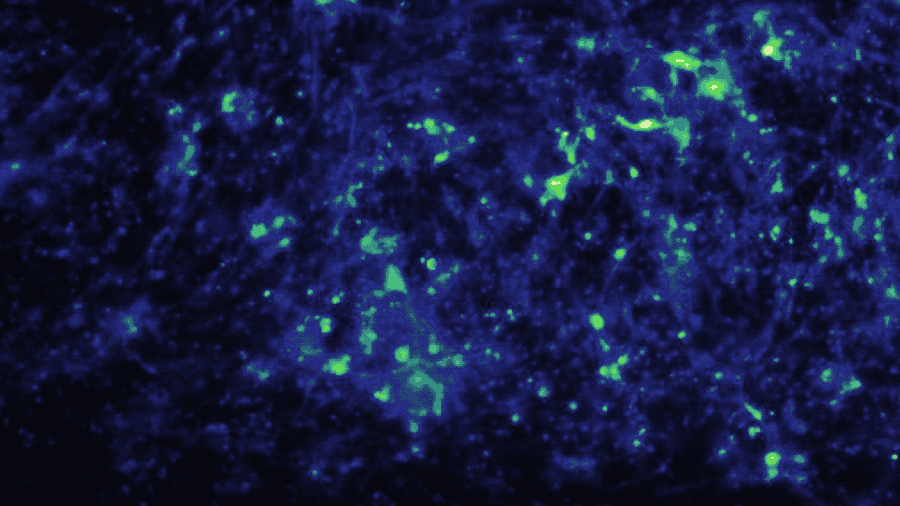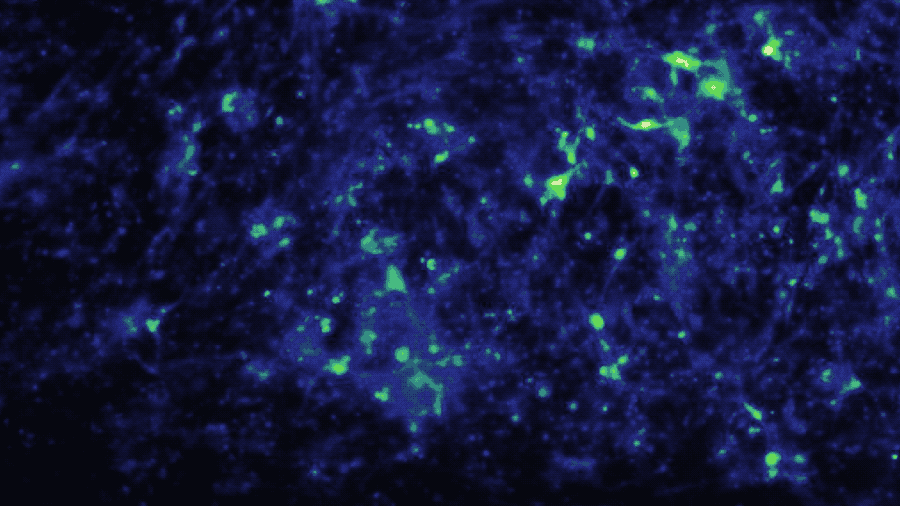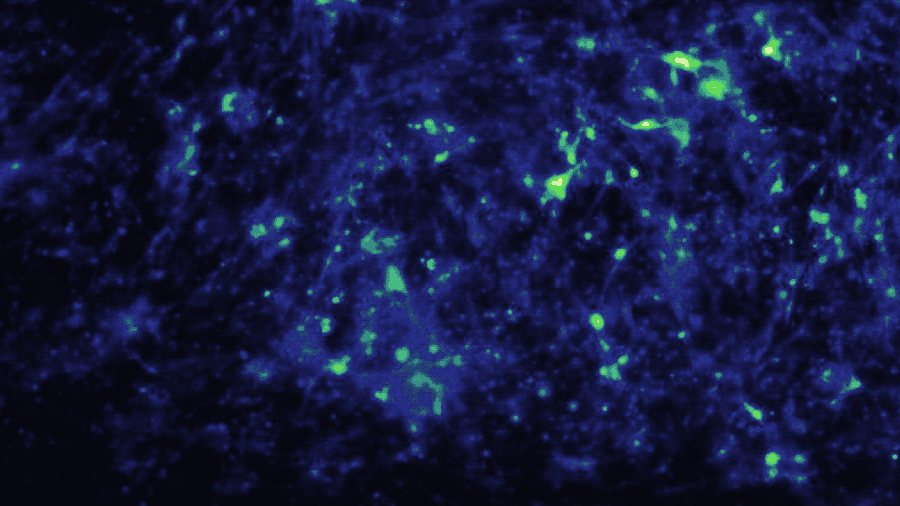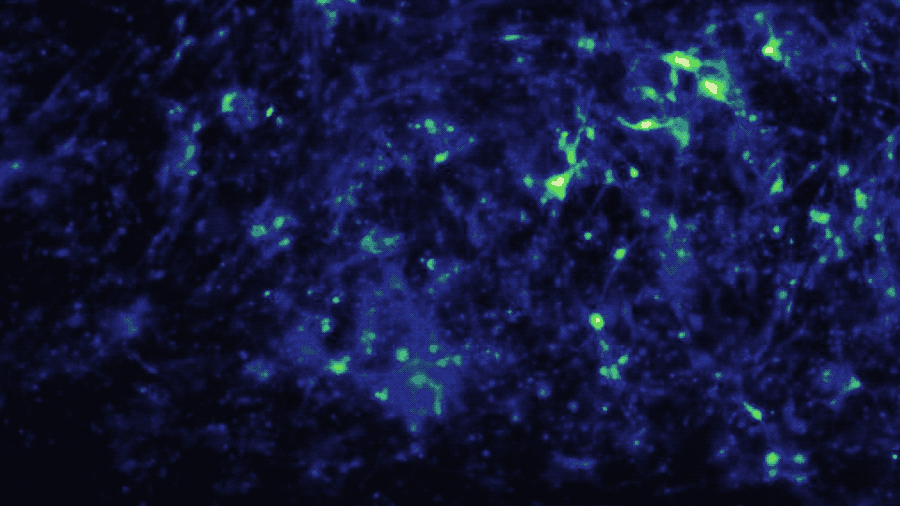
In a study published in iScience, the Brain-Chip (currently under development) has been applied to model neuroinflammation and its effect across the neurovascular unit. After exposure to an inflammatory stimulus (TNF-α) via the brain channel, the Brain-Chip reproduced key features of neuroinflammation. These include glial activation, proinflammatory cytokine secretion, neuronal loss, disruption of barrier integrity, and significant enrichment of inflammatory gene pathways. By comparison, a version of the Brain-Chip without microglia showed significantly decreased cytokine secretion, demonstrating their role in driving neuroinflammation.
The two-channel structure of the chip also enabled investigation of the role that route of administration has on neuroinflammatory response. Compared to brain channel administration, TNF-α administration
via the vascular channel resulted in significant gene expression differences of glial pathways implicated in astrocytic scarring, microglial phagocytosis, and destabilization of myelin.
A drug efficacy study was conducted with minocycline, an anti-inflammatory capable of crossing the BBB. By administering minocycline in the vascular channel, the inflammatory response was reduced, barrier integrity was maintained and proinflammatory cytokine secretion was reduced.
Taken together, this shows the potential for the Brain-Chip to be used in studying mechanisms of neuroinflammation and efficacy of anti-inflammatory drug candidates to prevent or treat cytokine-mediated inflammation and barrier disruption.
Our Offering
The Brain-Chip: As close to an in vivo human brain study as you can get
Whether you’re looking to more accurately assess barrier penetration, investigate mechanisms of neuroinflammation, or evaluate drug efficacy, you can improve the human relevance of your research with the Brain-Chip.
Benefits
Study the human neurovascular unit in a single model
Recreate diverse features of neuroinflammation
Measure drug transport across a BBB-like barrier
Assess efficacy of anti-neuroinflammatory drug candidates
Supported Organ Models
Brain-Chip
Study human physiology, disease, and drug effect in a comprehensive model of the neurovascular unit.