In the early 20th century, the scientific community was in an upheaval. Fueled by the advent of in vitro cell culture and the dawning acceptance of chromosome theory, the scientific community began to turn toward next-generation models, despite protests from scientists like renowned embryologist Ernest Everett Just.
“[Living matter] can never be divorced from its milieu,” Just wrote. “Our investigations of [life], however much for purposes of more refined and exact study… should never lose sight of the fact that the cell as organism is part with and of its environment.”1
“Holists” like Just believed that living things—be they cells or entire organisms—are as much products of their environment as they are of their internal matter. In contrast, reductionists took a more mechanical view, believing that reducing studies to the cells and chromosomes—life’s component parts—was sufficient to understand the nature and function of a living system.
For most of the last century, reductionism has dominated the life sciences to great effect. Two-dimensional (2D) cell culture models, such as petri dishes, are foundational tools for researchers studying human physiology. However, it is increasingly appreciated that cells in the human body exist in a dynamic microenvironment where fluid flow, signaling gradients, biomechanical forces, and other impactful features are in constant flux. True to Just’s warning, removing cells from this natural complexity can significantly alter their behavior, diminishing the translational value of these models. Such a realization has led to a burgeoning resurgence of holism, which has led to the rise of microphysiological systems.
Microphysiological Systems
“Microphysiological system” (MPS) broadly refers to any advanced in vitro system that allows cells to be cultured in a more natural, physiologically relevant environment. Unlike traditional 2D cell culture systems, wherein cells grow as monolayers atop plastic or glass dishes under static conditions, MPS expose cells to structural, material, or biophysical elements that replicate their native tissue physiology. Two of the most common MPS include:
- Organoids: Three-dimensional (3D) spherical clusters of stem-cell-derived and organ-specific cell types that are typically grown in an extracellular matrix (ECM), where they replicate certain structural and functional features of the organs they represent.
- Micropatterned tissue constructs: These are static systems that are typically designed to grow heterogeneous cell populations in a 3D scaffold. Microengineering technology allows for cells and their tissue-specific ECM proteins to be precisely positioned with a scaffold structure that closely resembles in vivo tissue.
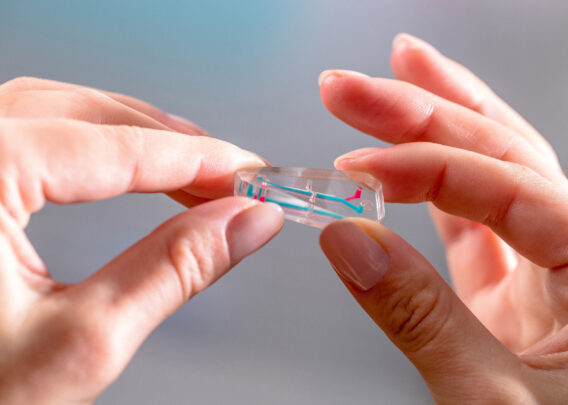
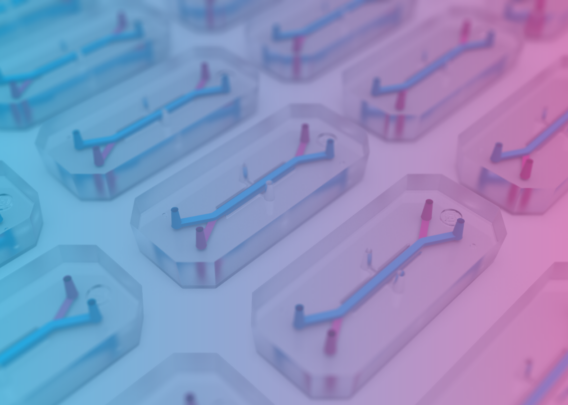
- Organ-on-a-Chip Technology: The most advanced type of MPS, Organ-Chips are 3D microengineered tissues that integrate microfluidic technology, enabling replication of fluid flow dynamics and other key biophysical forces that cells would naturally be exposed to in vivo.
By culturing cells in a more complex microenvironment that mimics their natural setting, MPS encourage cells to behave as they would in vivo, both in terms of transcriptomic profiles and phenotypic responses to various stimuli. Organ-Chips in particular have been demonstrated to recapitulate organ-level function for the liver, producing characteristic metabolites, metabolizing xenobiotics, and displaying signs of tissue damage in response to hepatotoxic drugs.2,3,4
As a result, the use of MPS in industries like pharma and academia has greatly increased in recent years, with applications ranging from the general study of human physiology to drug safety testing.
Stay Up to Date with the Emulate newsletter
Human Microphysiological Systems for Drug Development
Before candidate drugs can be tested in humans, they are typically first screened in highly reductionist model systems, such as 2D cell cultures, before being tested in animals. While a valuable process, the models that usher compounds towards the clinic are rife with limitations. Traditional culture systems lack physiological relevance and thus poorly predict how a compound will affect cells in a dynamic in vivo setting. Animal models provide the complexity of a living organism, but they are genetically and physiologically distinct from humans, which can greatly affect the perceived safety and efficacy of candidates.
These truths reiterate Just’s observation that living matter can never be divorced from its natural environment, and MPS are designed to provide this kind of environment for candidate compounds. Mounting evidence indicates that MPS are better able to predict how drug candidates will affect human tissues relative to both animal models and traditional 2D culture systems.3,4 As such, drug developers are increasingly integrating MPS into preclinical drug development pipelines.
As the adoption of human microphysiological systems for drug development continues to grow, the technology will likely further evolve towards a more holistic representation of human physiology. Defense Advanced Research Projects Agency (DARPA) has already invested5 in developing human-on-a-chip systems, wherein multiple different Organ-Chips are linked through microfluidics, emulating multi-organ systems. The advancing tide of ever more complex MPS systems promises to greatly improve the safety and productivity of modern drug development while moving scientific study toward an equilibrium balanced between reductionism and holism.
Frequently Asked Questions
Is There A Single Definition of “Microphysiological Systems”?
“Microphysiological” refers to two key aspects of microphysiological systems: (1) they are engineered with microscale precision using tools initially developed for the microchip industry; and (2) they are engineered to replicate natural human tissue physiology. However, there is currently no singular definition of microphysiological systems. However, the International Consortium for Innovation and Quality in Pharmaceutical Development’s MPS working group offers the following definition: “[Culture systems] that go beyond traditional 2D culture by including several of the following design aspects:
- A multi-cellular environment within biopolymer or tissue-derived matrix
- a 3D structure
- the inclusion of mechanical cues such as stretch or perfusion for breathing, gut peristalsis, flow
- incorporating primary or stem cell derived cells
- inclusion of immune system components6
Additionally, MPS can be separated into static and microfluidic systems based on the use (or lack thereof) of microfluidics. For example, organoids are static, whereas Organ-Chips are microfluidic.
Is There a Microphysiological Systems Conference?
The growing interest in microphysiological systems has resulted in numerous conferences dedicated to the subject. Recent examples include the EuroOCS 2022 and 2023 conferences organized by the European Organ-on-Chip Society; MPS World Summit 2023, which is organized by EuroOCS, the Johns Hopkins Center for Alternatives to Animal Testing, and the International MPS Society; and Global MPS Seminars, hosted by Emulate.
References (Formatted):
- Just, E. E. (1937). The Significance of Experimental Parthenogenesis for the Cell-biology of To-day. CYTOLOGIA, FujiiJubilaei(1), 540–550. https://doi.org/10.1508/cytologia.fujiijubilaei.540
- Jang, K.J., Otieno, M. A., Ronxhi, J., Lim, H.K., Ewart, L., Kodella, K. R., Petropolis, D. B., Kulkarni, G., Rubins, J. E., Conegliano, D., Nawroth, J., Simic, D., Lam, W., Singer, M., Barale, E., Singh, B., Sonee, M., Streeter, A. J., Manthey, C., & Jones, B. (2019). Reproducing human and cross-species drug toxicities using a Liver-Chip. Science Translational Medicine, 11(517), eaax5516. https://doi.org/10.1126/scitranslmed.aax5516
- Ingber, D. E. (2022). Human organs-on-chips for disease modelling, drug development and personalized medicine. Nature Reviews Genetics, 23, 467–491. https://doi.org/10.1038/s41576-022-00466-9\
- Ewart, L., Apostolou, A., Briggs, S. A., Carman, C. V., Chaff, J. T., Heng, A. R., Jadalannagari, S., Janardhanan, J., Jang, K.-J., Joshipura, S. R., Kadam, M. M., Kanellias, M., Kujala, V. J., Kulkarni, G., Le, C. Y., Lucchesi, C., Manatakis, D. V., Maniar, K. K., Quinn, M. E., & Ravan, J. S. (2022). Performance assessment and economic analysis of a human Liver-Chip for predictive toxicology. Communications Medicine, 2(1), 1–16. https://doi.org/10.1038/s43856-022-00209-1
- Wyss Institute to Receive up to $37 Million from DARPA to Integrate Multiple Organ-on-Chip Systems to Mimic the Whole Human Body. (2012, July 24). Wyss Institute. https://wyss.harvard.edu/news/wyss-institute-to-receive-up-to-37-million-from-darpa-to-integrate-multiple-organ-on-chip-systems-to-mimic-the-whole-human-body/
- Fabre, K., Berridge, B., Proctor, W. R., Ralston, S., Will, Y., Baran, S. W., Yoder, G., & Van Vleet, T. R. (2020). Introduction to a manuscript series on the characterization and use of microphysiological systems (MPS) in pharmaceutical safety and ADME applications. Lab on a Chip. https://doi.org/10.1039/c9lc01168d