Application
AAV Transduction & Toxicity in the Liver-Chip
Accelerate development of gene therapy vectors in a human-relevant liver model
Your challenge
Development of safe and efficient gene therapy vectors remains slow
Gene therapy holds enormous promise for inherited and acquired genetic diseases, yet progress remains slow. Developing safe and effective viral vectors is time consuming and challenging. Animal studies are slow, costly, and tightly regulated, while the limited complexity of conventional in vitro results in non-physiological response. These challenges have led not only to a slow pace of gene therapy approval, but also serious safety risks to patients.
our solution
A faster path to vector optimization
With the adeno-associated virus (AAV) transduction application for the Emulate Liver-Chip co-culture, you can rapidly iterate on AAV design in a human-relevant model of the liver sinusoid to accelerate vector optimization ahead of clinical trials.
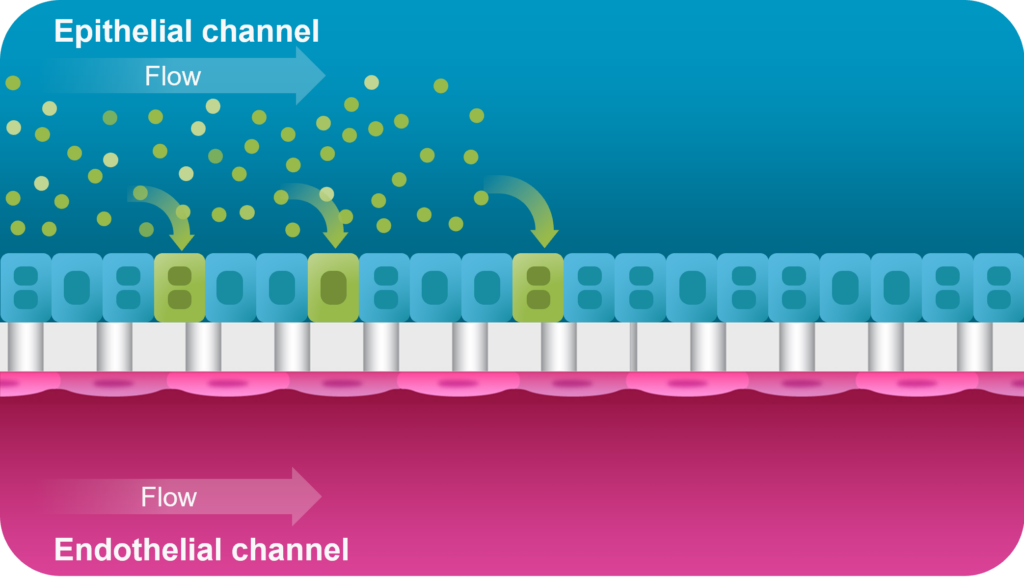
The Liver-Chip co-culture includes primary human hepatocytes and liver sinusoidal endothelial cells in the presence of tissue-specific extracellular matrix and media flow, resulting in enhanced hepatic functionality (albumin, urea, CYP450 activity) over conventional sandwich culture. With this application, you can administer your test AAV vector in the epithelial channel and monitor transduction and toxicity signals for up to seven days, enabling you to assess response over time.
The Liver-Chip is also available in quad-culture configuration, providing additional cellular complexity with the addition of Kupffer and stellate cells.
administer your test vector to:
Assess time- and concentration-dependent AAV transduction efficiency
Evaluate the toxicity of AAV-based gene therapy
Discriminate between the transduction efficiency of different AAVs

Supported Assays
transduction efficiency
Assess time- and concentration-dependent transduction
After 24 hours of AAV administration in the epithelial channel, transduction can be assessed over time with brightfield imaging.
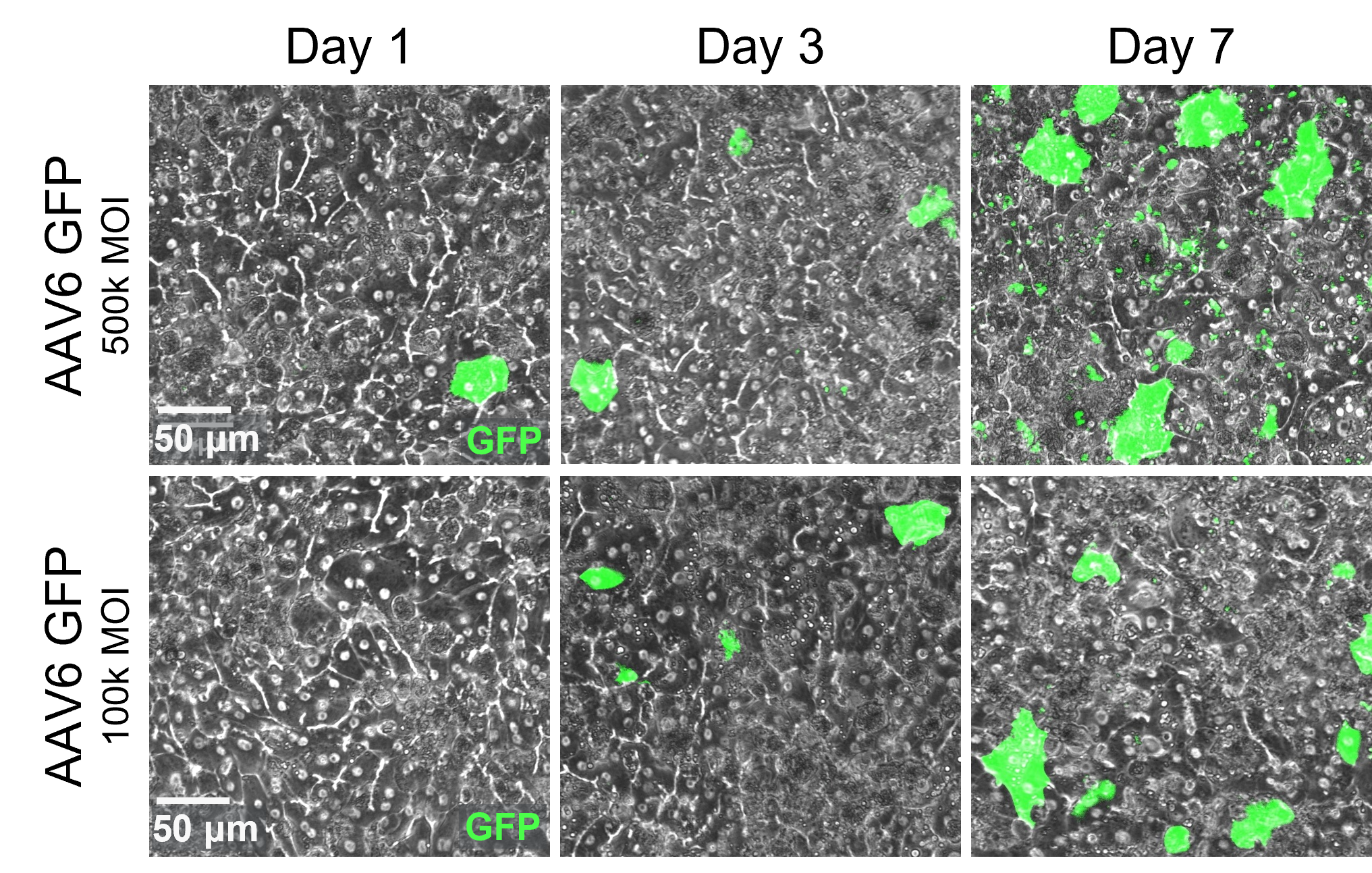
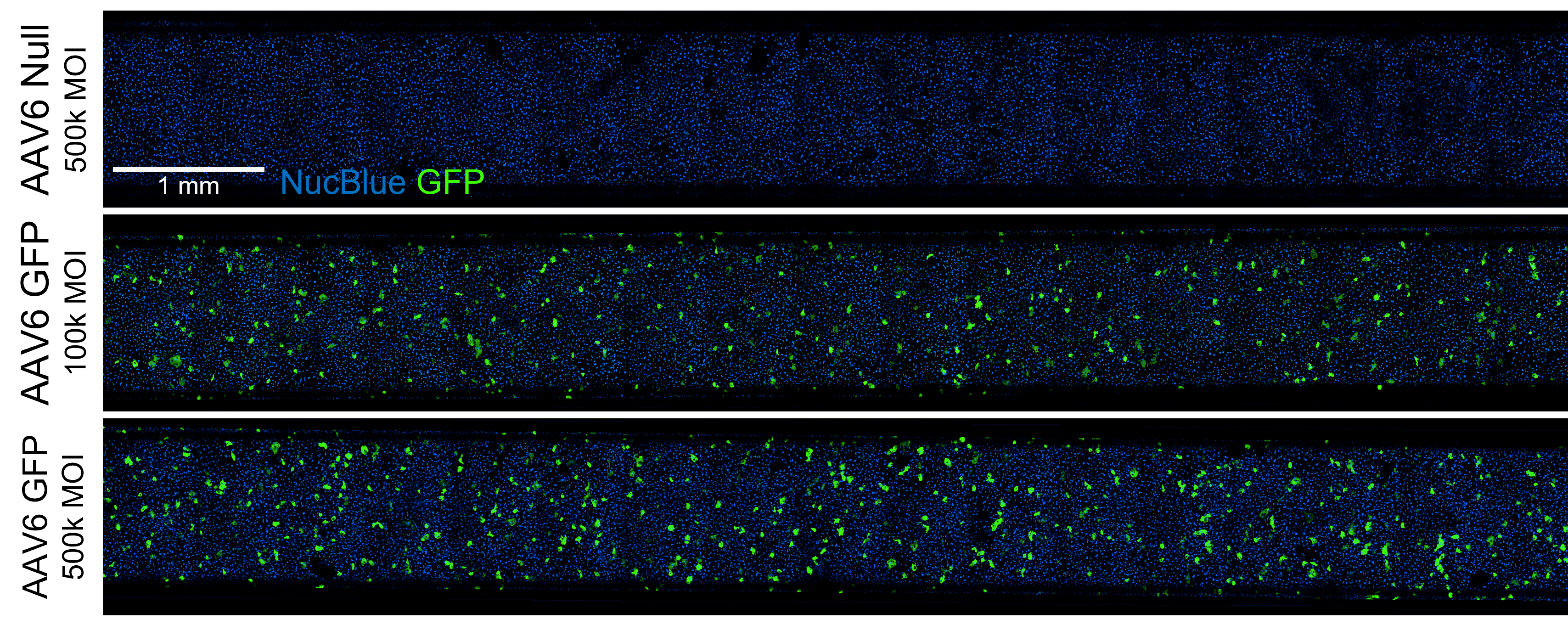
transduction efficiency
Full-chip fluorescent imaging enables qualitative assessment of vector transduction
Full-chip imaging can be performed as a terminal endpoint for greater confidence in transduction efficiency.
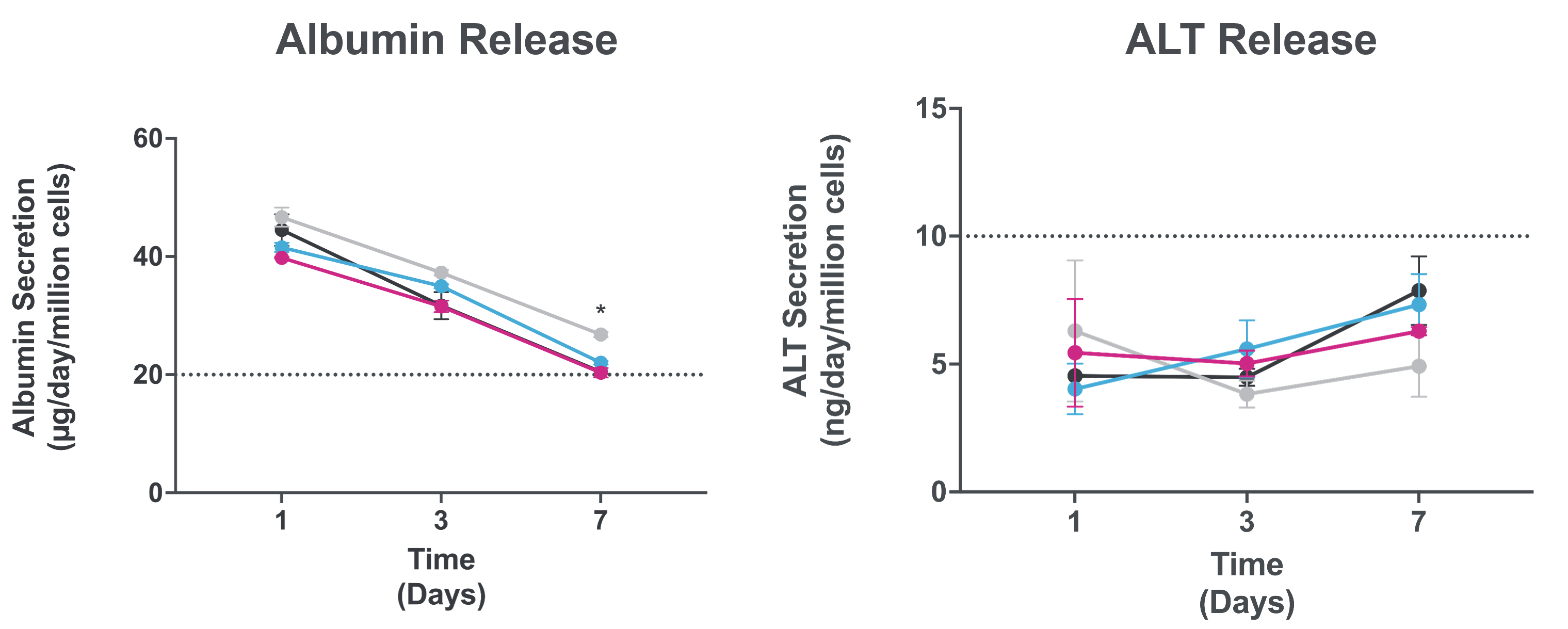
toxicity
Assess AAV vector toxicity through effluent analysis of key liver functional proteins
Measurement of albumin and ALT enable assessment of AAV toxicity risk to humans and avoid clinical translation failures due to species differences in toxicity.
Featured Resource
Application Note: Testing Transduction of AAV-Based Therapeutics on the Liver-Chip